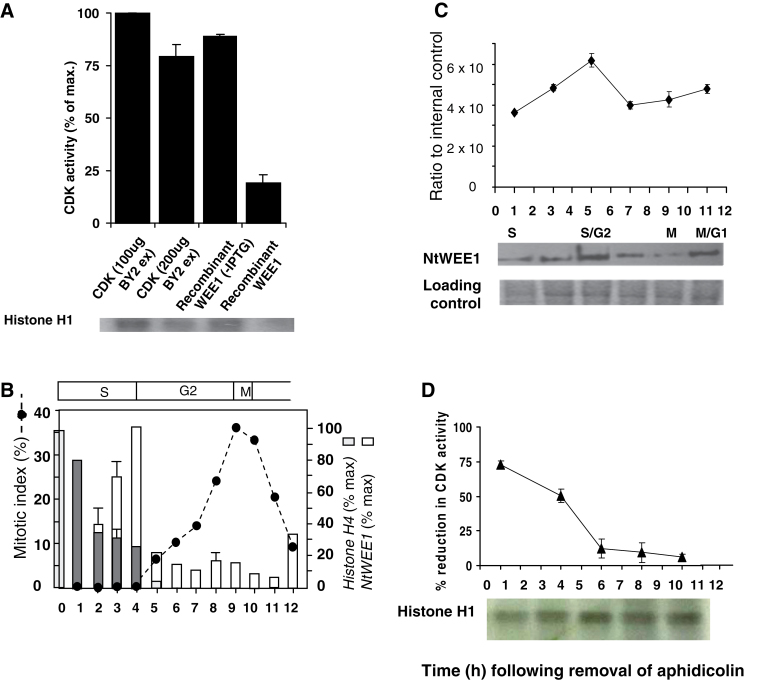
Fig. 1.
(A) Reduction in CDK activity elicited by the addition of recombinant NtWEE1. HIS6-NtWEE1 fusion protein, induced in E. coli (+IPTG) [compared with uninduced (–IPTG) control] was purified by affinity purification, and added to a kinase assay reaction containing CDKs (200 µg) purified from wild-type N. tabacum BY-2 cell culture protein extracts using p13SUC1 beads and histone H1 substrate; compared with the CDK alone (100 or 200 µg). A representative autoradiograph is shown below the histogram. The incorporation of 32P was assayed by quantification of the bands on the autoradiograph. (B–D) Cell cycle regulation of WEE1 mRNA, protein, and kinase activity in a synchronized wild-type N. tabacum BY-2 cell culture following removal of aphidicolin. (B) Mitotic index profile (dotted line) calculated as the sum of prophase, anaphase, metaphase, and telophase mitotic figures as a percentage of a minimum of 300 cells; mean histone H4 expression level (% max ±SE) (grey bars), mean NtWEE1 expression level (% max ±SE) (white bars), error bars absent when variation about the mean was <5% (n=3). The corresponding cell cycle phases are shown above the mitotic index graph. (C) Immunodetection of NtWEE1 protein extracted from synchrony samples (10 µg of protein per lane) and subjected to western blotting using the NtWEE1 antibody. The histogram displays mean (±SE) WEE1 protein levels as a ratio to an internal control (n=3). Representative western blot and a Coomassie stain loading control are shown below the histogram. (D) Inhibition of CDK activity by NtWEE1. The incorporation of 32P into histone H1 was assayed by quantification of the bands on the autoradiographs and expressed as the percentage reduction in CDK activity (± SE). CDK was pulled-down from 100 µg of BY-2 proteins. A representative autoradiograph is shown below the histogram.