Fig. 1.
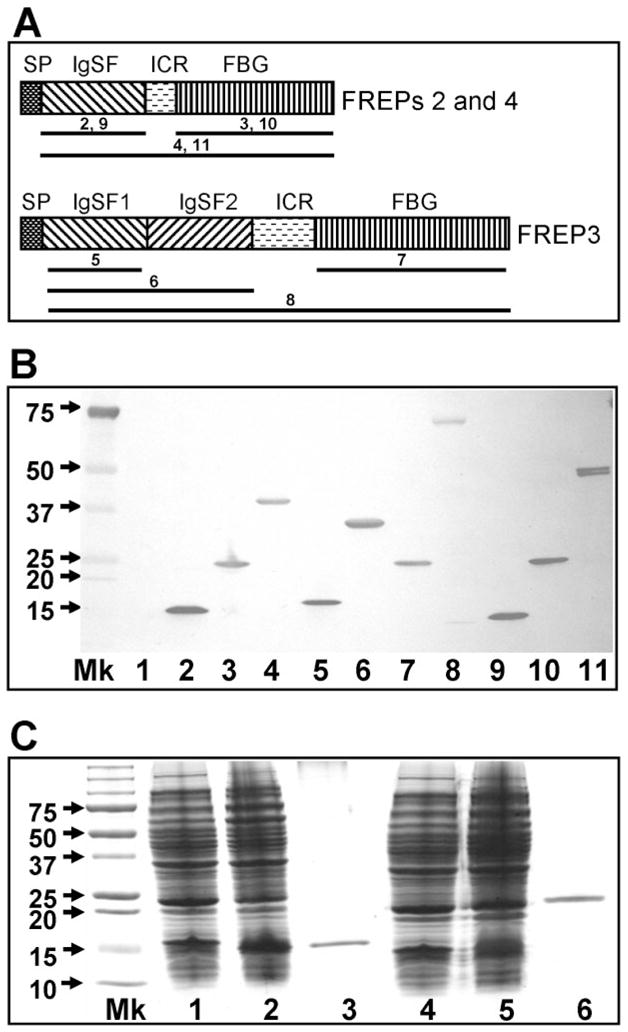
In vitro expression of full-length and truncated forms of FREPs. (A) Schematic diagram of gene products of FREPs with one IgSF domain (FREPs 2 and 4) or two IgSF domains (FREP3). SP, signal peptide; ICR, interceding region; IgSF, immunoglobin superfamily; FBG, fibrinogen. The regions underlined for each FREP were cloned into vectors and expressed in E. coli. The number above the line represents the number of the gel lane in (B). The diagrams are not drawn to scale. (B) Western blot showing expression of 10 rFREP proteins including truncated forms. Blots were probed with anti-6xhis monoclonal antibody because all rFREPs are tagged with 6 histamines at the C-terminus. Mk, protein marker; Lane 1 is loaded with control bacteria lacking expression constructs. Lanes are numbered as follows: (2) rIgSF-FREP2 (142 aa; pETbF2Ig-F+pETbF2Ig-R), (3) rFBG-FREP2 (210 aa; pETbF2FG-F+pETbF2FG-R), (4) rFREP2 (374 aa; pETbF2Ig-F+pETbF2FG-R), (5) rIgSF1-FREP3 (141 aa; pETbF3Ig1-F+pETbF2Ig1-R), (6) rIgSF1+2-FREP3 (301 aa; pETbF3Ig1-F+pETbF3Ig2-R), (7) rFBG-FREP3 (212 aa; pETbF3FG-F+pETbF3FG-R), (8) rFREP3 (648 aa; pETbF3Ig1-F+pETbF3FG-R), (9) rIgSF-FREP4 (141 aa; pETbF4Ig-F+pETbF4Ig-R), (10) rFBG-FREP4 (266 aa; pETbF4FG-F+pETbF4FG-R), and (11) rREPF4 (408 aa; pETbF4Ig-F+ pETbF4FG-R). The size of the peptide generated and the primer combination used for generation of the peptide are provided in parentheses. Primer sequences are listed in Table 1. (C) SDS-PAGE gel showing the expression and purification of IgSF and FBG domains of FREP4 from E. coli stained by Coomassie blue. Lane Mk is protein size markers; Lanes 1 and 4 are proteins expressed by E. coli lacking expression vectors; Lanes 2 and 5 are proteins expressed by bacteria transformed with expression vectors (lane 2, IgSF vector; lane 5, FBG vector); Lanes 3 and 6 show the purified rIgSF and rFBG proteins obtained using the nickel column.
