Fig. 5.
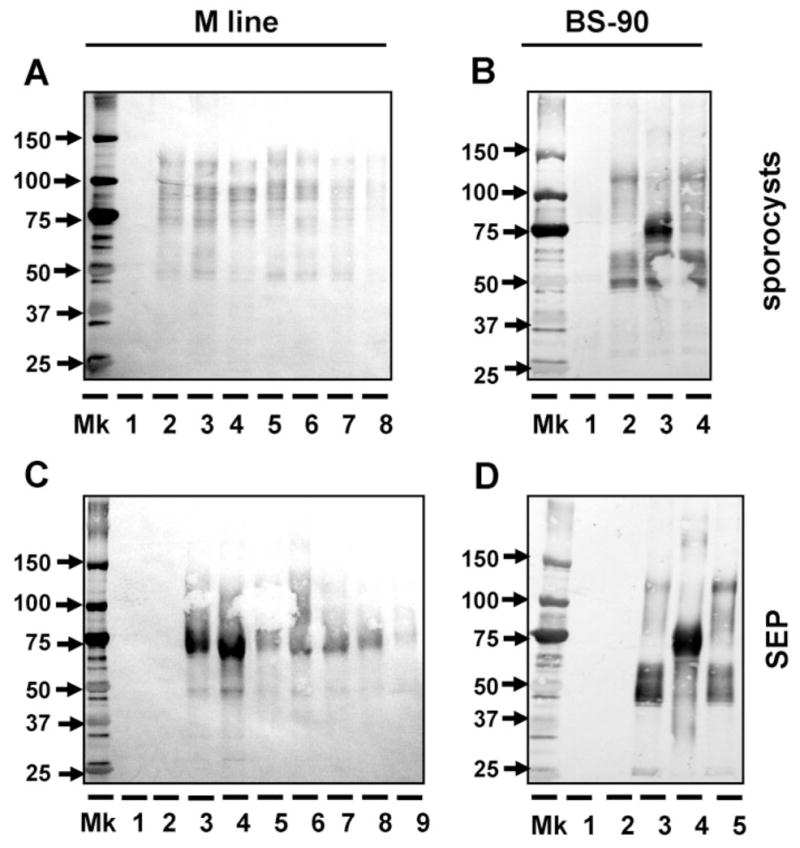
Use of anti-rFBG antibody to detect the binding of putative multiple FREPs from two snail strains (M line and BS-90) to E. paraensei sporocysts (A, B) and E. paraensei sporocyst SEPs (C, D). (A) Binding of M line plasma to E. paraensei sporocysts. Lane 1 is loaded with solubilized sporocysts. Lanes 2–8 are loaded with M line plasma components that bound sporocysts, from non-exposed controls snails (lane 2); snails exposed to E. paraensei (lane 3) or S. mansoni (lane 4); or snails injected with PBS (lane 5), Staph. aureus (lane 6), E. coli (lane 7) or S. cerevisiae (lane 8). All plasma was collected at 4 days post-infection or post-injection for all binding studies throughout this work. (B) Binding of BS-90 plasma to E. paraensei sporocysts. Lane 1 is loaded with sporocyst antigens only. Plasma components binding SEPs are derived from non-exposed control snails (lane 2), or snails exposed to E. paraensei (lane 3) or S. mansoni (lane 4). (C) Binding of M line plasma to SEPs. All SEPs used in this study was derived from E. paraensei sporocysts at 2–4 days post-transformation in vitro. Lane 1 received SEPs only. Lane 2 was loaded with half-strength 199-medium. Treatments for lanes 3–9 are the same, and in the same order, as described for lanes 2–8 in Figure 6A. (D) Binding of BS-90 plasma to SEPs. Lanes 1 and 2 were loaded the same as lanes 1 and 2 as described in the caption to Figure 5C. Treatments for lanes 3 to 5 are the same, and in the same order, as lanes 2 to 4 in Figure 5B.
