Abstract
Current therapies for immune-mediated inflammatory disorders in peripheral nerves are non-specific, and partly efficacious. Peripheral nerve regeneration following axonal degeneration or injury is suboptimal, with current therapies focused on modulating the underlying etiology and treating the consequences, such as neuropathic pain and weakness. Despite significant advances in understanding mechanisms of peripheral nerve inflammation, as well as axonal degeneration and regeneration, there has been limited translation into effective new drugs for these disorders. A major limitation in the field has been the unavailability of reliable disease models or research tools that mimic some key essential features of these human conditions. A relatively overlooked aspect of peripheral nerve regeneration has been neurovascular repair required to restore the homeostatic microenvironment necessary for normal function. Using Guillain-Barré syndrome (GBS) and chronic inflammatory demyelinating polyradiculoneuropathy (CIDP) as examples of human acute and chronic immune-mediated peripheral neuroinflammatory disorders respectively, we have performed detailed studies in representative mouse models to demonstrate essential features of the human disorders. These models are important tools to develop and test treatment strategies using realistic outcomes measures applicable to affected patients. In vitro models of the human blood-nerve barrier using endothelial cells derived by endoneurial microvessels provide insights into pro-inflammatory leukocyte-endothelial cell interactions relevant to peripheral neuroinflammation, as well as potential mediators and signaling pathways required for vascular proliferation, angiogenesis, remodeling and tight junction specialization necessary to restore peripheral nerve function following injury. This review discusses the progress we are making in translational peripheral neurobiology and our future directions.
Keywords: blood-nerve barrier, chronic inflammatory demyelinating polyradiculoneuropathy, endothelial cells, Guillain-Barré syndrome, mouse models, neuroinflammation, peripheral nerve, regeneration
Introduction
Peripheral nerve disorders (otherwise known as peripheral neuropathies) are a very common group of disorders characterized by axonal degeneration, demyelination or both in peripheral nerves. About 2–8% of the world’s population, including over 20 million Americans, is affected by peripheral neuropathies. Peripheral neuropathies can be acquired or inherited. Acquired causes are commonly due to trauma (e.g. carpal tunnel syndrome), metabolic/ endocrine disturbances (such as diabetes mellitus, thyroid disease), infections (such as leprosy, human immunodeficiency virus), drugs and toxins, nutritional deficiencies (such as vitamin B12 deficiency), cancer, primary autoimmune inflammatory disorders restricted to nerves and nerve roots, systemic autoimmune inflammatory disorders (such as systemic lupus erythematosus, rheumatoid arthritis) to name a few. A major consequence of peripheral neuropathies is the development of neuropathic pain, irrespective of the cause. [1–3] Neuropathic pain is potentially disabling and may affect over 1% of the United States population.[1, 4] Current treatments for peripheral neuropathies and neuropathic pain are non-specific and in many cases, partly effective at best and associated with considerable side effects.[1, 5, 6] The direct health care cost runs into several billion U.S. dollars per annum, excluding losses from reduced productivity.[7] Of particular interest to our research laboratory are the primary acute and chronic autoimmune inflammatory peripheral nerve disorders.
Guillain-Barré syndrome (GBS) is the most common cause of rapidly progressive, potentially life-threatening weakness in industrialized nations. GBS is a disorder that commonly develops days to weeks after certain bacterial or viral infections or minor trauma. This observation suggests an autoimmune response related to molecular mimicry of peripheral nerve antigens, including gangliosides.[8–10] GBS is characterized by demyelination, axonal degeneration or both that is restricted to nerve roots and peripheral nerves. This causes weakness, loss of sensation and cardiovascular instability that may progress to respiratory failure and death.[11, 12] The likelihood of permanent disability or death is related to the severity of weakness.[8–10] Based on 2004 estimates, the total average annual cost of GBS to the United States was ~$1.7 billion.[13] The most common variant of GBS, acute inflammatory demyelinating polyradiculoneuropathy (AIDP), is associated with nerve root and peripheral nerve infiltration by hematogenous monocytes/ macrophages, T-cells and B-cells/ plasma cells. There is pathological evidence of cell and humoral-mediated demyelination and axonal injury.[8–10] Although the precise trigger for autoimmune attack is unknown, inhibition of leukocyte infiltration at the early stages could reduce the extent of demyelination and axonal injury, improving patient outcomes in GBS. Despite advances in molecular biology and genetics, there have been no new GBS treatments or significant improvements in patient outcomes over the last 20 years.[10, 14]
Chronic inflammatory demyelinating polyradiculoneuropathy (CIDP) is an acquired immune-mediated disorder affecting multiple nerve roots and peripheral nerves that reaches maximal severity >8 weeks after symptom onset.[5, 15] This is a clinically heterogeneous disorder and is commonly misdiagnosed or under-recognized. Prevalence is estimated at ~2–8 per 100,000 persons.[5, 15] CIDP may account for ~14% of disabling neuropathies in patients older than 65 years.[16] CIDP has a relapsing-remitting, steady or step-wise progressive course that commonly results in long-term disability (related to the degree of associated axonal injury).[15, 17] CIDP is pathologically characterized by multifocal, inflammatory demyelination that involves spinal nerve roots and nerves, with lesions extending throughout the peripheral nervous system. Macrophages and T-lymphocytes are commonly seen in affected nerve biopsies, supporting an important pathogenic role for leukocyte infiltration.[17, 18] Although the precise trigger for the autoimmune attack is also unknown, preventing early leukocyte infiltration could reduce the extent of demyelination and axonal injury, improving patient outcomes in CIDP.[6]
In order to fully understand how to effectively develop strategies to treat peripheral neuropathies, an appreciation of how peripheral nerves are organized and their vascular supply is needed. Peripheral nerves have a unique vascular network due to their anatomical compartmentalization. Peripheral nerves can be divided into an external epineurium, inner perineurium and innermost endoneurium. Axons and their supportive glial cells, called Schwann cells are located within the endoneurium. In order to maintain normal axonal function (transmission of action potentials to or from the central nervous system), the endoneurial microenvironment must be strictly regulated. The restricted microenvironment is regulated by specialized endoneurial microvascular endothelial cells and perineurial myofibroblasts.[19–23]
These cells are characterized by intercellular tight junctions, the lack of fenestrations and few 50–100 nm pinocytic vesicles. Macrovessels in the epineurium lack tight junctions and have numerous fenestrations.[19, 20] This implies that solutes or macromolecules in epineurial blood circulation may ‘leak’ out from these vessels prior to entry into the endoneurium. Endoneurial endothelial cells restrict the passive diffusion of solutes and macromolecules and leukocyte entry from the blood circulation into the endoneurium, while the perineurial cells restrict passive diffusion from the epineurial interstitial space into the endoneurium.[21–25] Endoneurial endothelial cells can be considered the true “blood-nerve barrier”, as these cells are in direct contact with circulating blood. The blood-nerve barrier is second only to the blood-brain barrier in terms of restrictive endothelial barrier function in mammals.[23, 26, 27]
A feature relevant to peripheral neuropathies is the interaction between soluble and cellular components of the blood circulation and myelinated or unmyelinated axons or both within the endoneurium through the blood-nerve barrier. Very little is known about human blood-nerve barrier development, maturation, tight junction specialization and response to injury. Significant progress has been made in understanding mechanisms of axonal growth, [28] and Schwann cell de-differentiation, proliferation and differentiation into myelin-producing cells during normal development and following nerve injury.[29–31] Structural and functional alterations at the blood-nerve barrier have been implicated in the pathogenesis of peripheral neuropathies.[5, 6, 32–35] Restoration of blood-nerve barrier function could accelerate peripheral nerve recovery from injury by re-establishing the required endoneurial microenvironment needed for axonal regeneration, remyelination and subsequent signal transduction.
Translational efforts in peripheral neuroinflammation and neurovascular repair have been hampered by a lack of patient nerve samples for extensive exploratory studies, the lack of human blood-nerve barrier models to study pathogenic inflammatory or repair mechanisms in vitro and limitations in animal models required to evaluate possible pathogenic mechanisms in vivo before clinical drug trials are planned.[14] In order to overcome these hurdles, we have characterized reliable mouse models of GBS and CIDP and developed an in vitro model of the human blood-nerve barrier. [36–38] Our purpose is to study and modulate key pathologic features of peripheral neuroinflammation and neurovascular injury guided by human observational data.
Experimental models and translational paradigms
In vivo models of acute peripheral neuroinflammation: Experimental Autoimmune Neuritis
Experimental autoimmune neuritis (EAN) is an animal model for GBS, and is induced by the administration of peripheral nerve myelin or myelin proteins/peptides to susceptible animals with consequential peripheral nerve demyelination, axonal degeneration or both. [6] The most commonly cited model, Lewis rat EAN, resembles the human AIDP variant of GBS and has provided some insights relevant to GBS immunopathogenesis. Despite these advances, there has been a failure to translate these observations towards new therapies for GBS. It is imperative to use models that closely resemble the human disease and design experiments guided by human observational data. Drug administration prior to the development of clinically observable disease in rodents does not apply to the reality of clinical practice in affected patients. Realistic outcome measures based on clinical and electrophysiological features are required to ensure that treatment effects are significant enough to be applicable to humans. Due the widespread availability of reagents and genetic knockouts in mice, as well as similarities between human and mouse immune responses, murine EAN models would aid advance knowledge on GBS immunopathogenesis necessary to elucidate specific treatment targets. Unfortunately, most mouse strains are relatively resistant to EAN induction. [6]
A severe mouse EAN model using female SJL/J mice was described by Calida et al in 2000, [39] and we demonstrated that this model recapitulates essential neurobehavioral, electrophysiological and histopathological features of the AIDP variant of GBS (Figure 1). We termed this model severe murine EAN (sm-EAN). Following disease induction with bovine peripheral nerve myelin, with pertussis toxin and recombinant mouse interleukin-12, mice develop progressive weakness starting 7–10 days post-induction, reaching peak weakness by day 26–32. The earliest observed deficit is tail weakness, following by forelimb or hind limb weakness. At peak severity, there is severe weakness in both the hind and forelimbs. We use a 6-point neuromuscular severity scale to semi-quantitatively describe the neurobehavioral changes observed in sm-EAN; 0: No weakness, 1: limp tail or tail weakness, 2: mild-to-moderate fore or hind limb weakness, 3: severe fore or hind limb weakness, 4: mild-to-moderate fore and hind limb weakness and 5: severe fore and hind limb weakness.[37] As observed in AIDP, affected mice at peak severity demonstrate electrophysiological parameters consistent with demyelination (reduced conduction velocities, prolonged motor action potential durations [temporal dispersion], conduction block between distal and proximal responses) and axonal loss (reduced distal compound motor action potential amplitudes) in the dorsal caudal tail and sciatic nerves.[37]
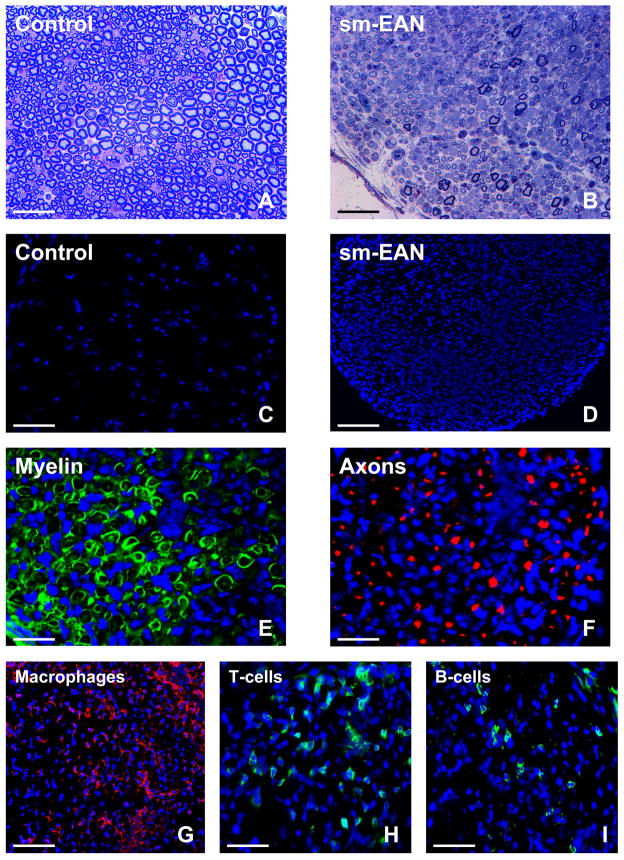
Figure 1. Histopathological features of sm-EAN.
Representative toluidine-blue stained, basic fuchsin counterstained photomicrographs of 1 μm glutaraldehyde fixed, osmium tetroxide post-fixed plastic-embedded mouse sciatic nerve axial sections show the normal distribution of myelinated axons in control female SJL/J mice (A) in contrast to the severe demyelination and reduction in axonal density coupled with mononuclear cell infiltration in sm-EAN at peak severity (B). The extent of intense mononuclear cell infiltration in sm-EAN is depicted by the 4′,6-diamidino-2-phenylindole (DAPI)-stained photomicrographs of 10 μm frozen sciatic nerve axial sections (D) compared to unaffected controls (C). Sm-EAN is associated with demyelination, as demonstrated by fragmentation of Schwann cell S100β (green) immunoreactivity (E), and axonal loss depicted by reduced neurofilament-H (red) immunoreactivity (F) as described in nerve biopsies of human AIDP. Mononuclear leukocyte infiltration consists predominantly of F4/80+ macrophages (red immunoreactivity, G), followed by T-cells (H) and B-cells (I), depicted by green immunoreactivity. Blue depicts nuclei (DAPI stain). Kindly refer to [37] for a detailed histopathological and electrophysiological characterization of sm-EAN. Magnification bars: A, B, G = 50 μm, C, D = 100 μm, E, F= 25 μm, H, I = 35 μm.
Increased expression of pro-inflammatory chemokines has been described in the peripheral nerves and cerebrospinal fluid of patients with GBS compared to controls. [40–43] We have demonstrated up-regulation in specific pro-inflammatory chemokine ligand-receptor pairs (CCL2-CCR2, CCL5-CCR1/CCR5 and CXCL10-CXCR3) in the sciatic nerves of affected mice at peak severity relative to unaffected controls. [44] We demonstrated CCL2 expression by Schwann cells and CCR2 on endoneurial macrophages implying a role for CCL2-CCR2 in macrophage-induced demyelination in sm-EAN [44] as suggested by observational studies in human nerve biopsies.[42] Macrophage-induced demyelination is a characteristic hallmark of AIDP.
CCL5 was expressed by axons, with CCR1 and CCR5 predominantly expressed on endoneurial macrophages [44], suggesting a role for these chemokine ligand and receptors in secondary axonal degeneration in this model. Secondary axonal degeneration is also commonly observed in pathologic AIDP nerve biopsies, inferred from electrodiagnostic studies and is a major cause of disability. Interestingly, CXCL10 was expressed by endoneurial endothelial cells and within the endoneurial space, associated with CXCR3-expressing T-cells [44]. This observation implies a role for CXCL10-CXCR3 in CD4+ T-helper 1-cell infiltration across the blood-nerve barrier and retention within the endoneurium in peripheral neuroinflammation. CXCL10 had been implicated in GBS pathogenesis based on sural nerve biopsy data from affected patients. [40]
Chemokine receptors are G-protein coupled receptors, a family of receptors to which over 50% of known drugs directly target. [45, 46] Although there is redundancy in chemokine-mediated signaling, the sm-EAN model provides a robust tool to determine the efficacy of chemokine receptor antagonists in acute peripheral nerve inflammation in vivo using neurobehavioral and electrophysiological outcomes measures applicable to human clinical trials and subsequent practice. In this regard, we are currently evaluating the role of CCL2-CCR2 and CXCL10-CXCR3 signaling pathways in the immunopathogenesis of sm-EAN with translational potential to AIDP.
Integrin-dependent leukocyte adhesion to microvascular endothelium is an essential aspect of the multi-step paradigm of leukocyte infiltration into inflamed tissues.[47, 48] The efficacy of Natalizumab (humanized mouse anti-α4 integrin antibodies) in treating relapsing-remitting multiple sclerosis and inflammatory bowel disease provides impetus to evaluate the role of integrin signaling in peripheral neuroinflammation. Guided by our recent human in vitro work, [49] we demonstrated significant expression of αM-integrin (CD11b) on endoneurial F4/80+ macrophages, CD3+ T-cells and CD19+ B-cells at peak severity relative to unaffected controls in sm-EAN, suggesting a role for αM-integrin-dependent signaling in peripheral neuroinflammation (Figure 2, unpublished observations). Leukocyte αM-integrin interacts with intercellular adhesion molecule-1 (ICAM-1) constitutively expressed by vascular endothelial cells and upregulated during inflammation, [49–52] providing targets for specific blockade in sm-EAN that may have translatable relevance to human AIDP. Inhibition of pathogenic leukocyte infiltration into peripheral nerves is an attractive target in peripheral neuroinflammation as the drugs would be efficacious in systemic circulation and would not require permeability across the restrictive blood-nerve barrier to carry out their pharmacologic effects.
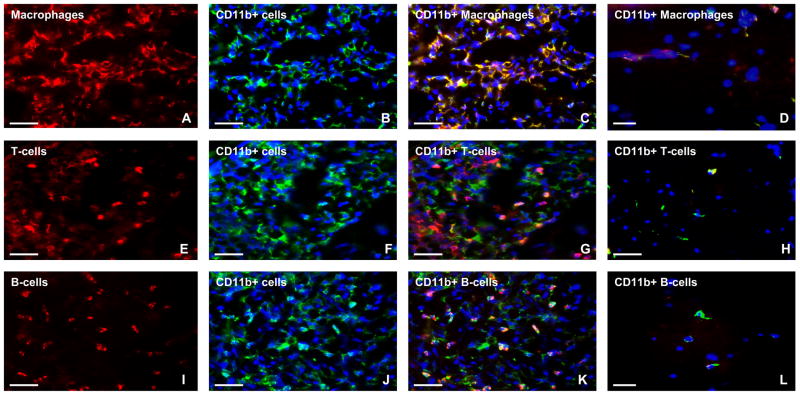
Figure 2. Expression of αM-integrin (CD11b) in sm-EAN.
Representative indirect fluorescent immunohistochemistry photomicrographs of 15 μm frozen sciatic nerve axial sections show intense infiltration of CD11b+ macrophages (A–C), T-cells (E–G) and B-cells (I–K) in sm-EAN compared to unaffected controls (D, H and L respectively). Red immunoreactivity represents infiltrated F4/80+ macrophages (A), CD3+ T-cells (E) and CD19+ B-cells (I), while green immunoreactivity represents leukocyte αM-integrin expression on infiltrated mononuclear leukocytes (B, F and J: blue [DAPI-stain] depicts nuclei). Yellow or orange immunoreactivity represents αM-integrin-positive leukocyte subpopulations (C, G, K) in sm-EAN. Comparatively, most of the αM-integrin-positive leukocytes are macrophages (C), followed by B-cells (K), then T-cells (G). Rare αM-integrin-positive macrophages and T-cells (yellow/orange immunoreactivity) are seen in control nerves (D, H), without B-cell expression (I). Images were initially taken using the same exposure times and digitally merged. Magnification bars represent 50 μm.
In vivo models of chronic peripheral neuroinflammation: Spontaneous Autoimmune Peripheral Polyneuropathy
Robust animal models of CIDP have been a challenge due to variability in disease induction, lack of chronic persistence or unpredictable number of relapses and remissions. [6] CIDP and GBS occur spontaneously in humans, in contrast to the aggressive induction protocols using whole myelin or myelin proteins/ peptides and adjuvants required to induce specific immune responses and increase vascular permeability in animal models. The heterogeneity of the human disorder also provides a challenge in demonstrating that an animal model adequately represents essential features of CIDP necessary for translational drug studies. Salomon et al described a model of chronic peripheral nerve inflammation called spontaneous autoimmune peripheral polyneuropathy (SAPP) that developed in co-stimulator B7-2 (CD86) deficient non-obese diabetic mice after 20 weeks of age, with 100% disease penetrance in females, associated with normoglycemia.[53] This group subsequently demonstrated that SAPP is an interferon-γ dependent, CD4+ T-cell mediated disorder with humoral and cell-mediated immune responses targeted against myelin protein zero, the most abundant protein in peripheral myelin.[54] Using a myelin protein zero-specific T-cell receptor transgenic mouse bred unto a recombination activation gene knockout background, fulminant neuritis developed, associated with a reduction in antigen-specific CD4+ Foxp3+ regulatory T-cells.[54] We demonstrated that SAPP recapitulates essential neurobehavioral, electrophysiological and histopathological features of untreated severe unremitting CIDP between 20–40 weeks of age in affected female NOD mice [36] (Figure 3).
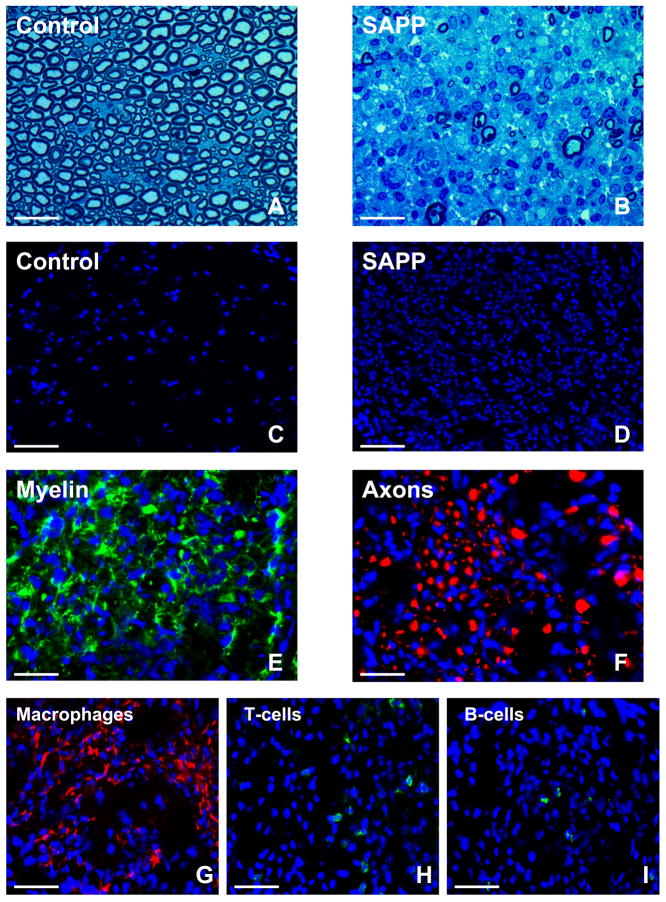
Figure 3. Histopathological features of SAPP.
Representative toluidine-blue photomicrographs of 1 μm glutaraldehyde fixed, osmium tetroxide post-fixed plastic-embedded mouse sciatic nerve axial sections show the normal distribution of myelinated axons in control B7-2 deficient non-obese diabetic mice (A) in contrast to the severe demyelination and reduction in axonal density coupled with mononuclear cell infiltration observed in SAPP at 30 weeks of age (B). The extent of intense mononuclear cell infiltration in SAPP is depicted by the DAPI-stained photomicrographs of 10 μm frozen sciatic nerve axial sections (D) compared to unaffected controls (C). SAPP is associated with demyelination, as demonstrated by the loss of the normal honeycomb Schwann cell S100β (green) immunoreactivity (E), and axonal loss/ fragmentation depicted by reduced density and spotty/ filamentous neurofilament-H (red) immunoreactivity (F) as described in severe cases of human CIDP. Mononuclear leukocyte infiltration consists predominantly of F4/80+ macrophages (red immunoreactivity, G). Fewer T-cell (H) and B-cell (I) infiltrates are seen, depicted by green immunoreactivity. Blue depicts nuclei (DAPI stain). Kindly refer to [36] for a detailed histopathological and electrophysiological characterization of SAPP. Magnification bars: A, B, E, F = 25 μm, C, D = 75 μm, G, H, I = 35 μm.
Our longitudinal studies showed that SAPP was associated with slowly progressive severe weakness in hind and forelimbs without significant recovery after 30 weeks of age. Based on these observations, we concluded that SAPP is a chronic progressive, inflammatory, predominantly demyelinating polyneuropathy with secondary axonal degeneration and cumulative axonal loss over time, as described in many cases of human CIDP.[36] In the early stages of disease, hematogenous leukocyte infiltration was associated with demyelination without axonal degeneration/loss. This raised the possibility that demyelinated axons are more susceptible to injury in chronic peripheral neuroinflammation. This preclinical model provides an opportunity to test whether inhibition of chronic peripheral nerve leukocyte infiltration, demyelination or both could prevent subsequent axonal loss, with direct relevance to human CIDP, where disability correlates to the extent of axonal compromise [17, 55].
Detailed knowledge of the natural history of SAPP also provides information needed to design potentially translatable therapeutic strategies with outcome measures relevant to human CIDP. The observation that SAPP disease progression was most rapid between 20–24 weeks (associated with electrophysiological hallmarks of demyelination) and reached a plateau by 31 weeks of age suggests that treatments should be administered following clinically appreciable disease onset, but prior to maximum severity after which irreversible axonal injury may have occurred. We are currently studying the role of the pro-inflammatory alternatively spliced variant of fibronectin derived from the type II connecting segment, called fibronectin connecting segment-1, in hematogenous leukocyte trafficking in this chronic peripheral neuroinflammation model as a means of discovering more targeted small molecular antagonists for CIDP and related disorders. SAPP also provides a tool to further study mechanisms of chronic persistent peripheral neuroinflammation and failure of peripheral nerve immune tolerance as a means to further understand why certain CIDP patients may become refractory to immune-modulatory therapy.
In vitro models of the human blood-nerve barrier: peripheral neuroinflammation
Human nerve biopsies provide useful information about the phenotypic characteristics of peripheral nerves in health and disease that can guide functional studies. In order to study functional mechanisms at the human blood-nerve barrier, we isolated primary endoneurial endothelial cells from sciatic nerves obtained at autopsy from recently decedent individuals and demonstrated retention of molecular and biophysical properties consistent with restrictive barrier-forming endothelial cells.[38] These include expression of vascular endothelial markers, specialized transporters, cellular adhesion molecules, tight junction associated proteins (including the endothelial-specific claudin-5) and high transendothelial electrical resistance and low permeability to a large macromolecule, dextran-70. [38]
Phenotypic and functional differences between microvascular and macrovascular endothelial cells from the same tissue and differences between endothelial cells from different tissues and species provided the rationale to develop an in vitro model of the human blood-nerve barrier to specifically study determinants and signaling mechanisms relevant to human peripheral neuroinflammation. We developed a dynamic real-time leukocyte trafficking assay using primary human endoneurial endothelial cells based on time-lapse video microscopy [49]. Guided by some knowledge on cytokine and adhesion molecule expression in sural nerves biopsies from GBS patients and estimated capillary hemodynamics, [41, 56–58] we utilized this model to demonstrate an important role of αM-integrin-ICAM-1 interactions in pathogenic untreated GBS (AIDP-variant) patient peripheral blood mononuclear leukocyte adhesion at the human blood-nerve barrier in vitro.[49]
Importantly, function neutralizing antibody blockade of αL-integrin (CD11a: the other known counterligand for ICAM-1) was not as effective as αM-integrin inhibition, despite its ubiquitous expression by GBS patient-derived mononuclear leukocytes. Monoclonal antibodies against αM-integrin were more potent than human immunoglobulin (a currently approved treatment) in modulating pathogenic leukocyte trafficking in this model. Our work also demonstrated basal chemokine and cellular adhesion molecule expression by the blood-nerve barrier with significant up-regulation or de novo expression of chemokines implicated in innate (neutrophil and monocyte recruitment) and proinflammatory adaptive immune responses (T-helper 1 and T-helper 17), and increased expression of cellular adhesion molecules (including ICAM-1 described in GBS affected endoneurial microvessels) following physiological cytokine treatment with tumor necrosis factor-α and interferon-γ. [49]
In addition to the differential regulation of αM-integrin expression on subpopulations of untreated GBS patient-derived peripheral blood mononuclear cells compared to healthy controls (suggesting a potential role for αM-integrin as a biomarker of AIDP disease state), our work also suggests a more significant role for the inflammatory state of the blood-nerve barrier endothelium than systemic leukocyte activation state in pathogenic leukocyte trafficking in vitro.[49] Coupled with preliminary observations of αM-integrin expression in sm-EAN sciatic nerves, these in vitro observations could potentially translate towards a more specific targeted anti-inflammatory therapy for GBS. The flow-dependent human blood-nerve barrier model, despite the known limitations of in vitro assays, provides a very useful tool to evaluate signaling pathways and potential new drugs that could target pathogenic hematogenous leukocyte trafficking into peripheral nerves during immune-mediated inflammation or nerve injury. We are currently using this model to study the role of fibronectin connecting segment-1, corticosteroids and human immunoglobulin on CIDP patient-derived leukocyte trafficking at the blood-nerve barrier in vitro.
In vitro models of the human blood-nerve barrier: neurovascular repair
In contrast to the central nervous system, peripheral nerves have regenerative potential. Research efforts have focused on axonal regeneration and directed migration towards peripheral targets, as well as axonal remyelination by Schwann cells following peripheral nerve injury. There is recent evidence demonstrating an important role for intraneural angiogenesis during regeneration in rodent models cerebral and peripheral nerve injury.[59–63] Restoration of the endoneurial microenvironment by the blood-nerve barrier and perineurium may be essential not only for axonal regeneration, but for normal axonal transmission. We sought to determine the determinants and potential signaling pathways required to restore human blood-nerve barrier function in vitro following injury.
Using serum withdrawal for 48 hours to non-specifically induce endothelial cell detachment from the confluent blood-nerve barrier in vitro, we demonstrated an important role for exogenous glial-derived neurotrophic factor (GDNF) in restoring blood-nerve barrier biophysical properties via up-regulation in its receptor, GFRα1 and activation of RET-tyrosine kinase signaling pathways.[64] We determined that there was some redundancy in vitro, as other mitogens had a less robust effect and required higher molar concentrations. We also observed a minor partial role for cyclic-adenosine monophosphate-protein kinase A-dependent signaling in this process. These observations implied that restoration of BNB resistance and permeability characteristics may be biologically essential in vivo. Interestingly, GDNF did not significantly up-regulate adherens or tight junction associated proteins (apart from a small increase in claudin-5 protein), or modulate claudin-5 tyrosine phosphorylation (implicated in increased permeability brain-derived mammalian capillary endothelial cells in vitro)[65] but induced F-actin cytoskeletal filaments that resulted in more continuous intercellular contacts that provided the necessary scaffold for adherens and tight junction formation between adjacent endothelial cells, associated with fewer intercellular gaps.[64]
GDNF has been implicated in the survival of peripheral autonomic and sensory neurons, as well as spinal cord motor neurons that form motor axons. Schwann cells are the major source of GDNF in peripheral nerves. [66–74] We infer from our study that Schwann cells may be responsible to some extent in human blood-nerve barrier recovery following peripheral nerve injury via paracrine secretion that directly acts on GFRα1-expressing endoneurial endothelial cells.[64] Using continuous electrical cell impedance sensing, ongoing work implicates mitogen associated protein kinase-dependent signaling downstream of RET-tyrosine kinase in GDNF-mediated blood-nerve barrier recovery following serum withdrawal in vitro (Figure 4). We are currently designing experiments to study the role of GDNF in blood-nerve barrier restoration following peripheral nerve injury in mice using conditional knockouts, as well as evaluating its effect on other biophysical properties of the human blood-nerve barrier in vitro.
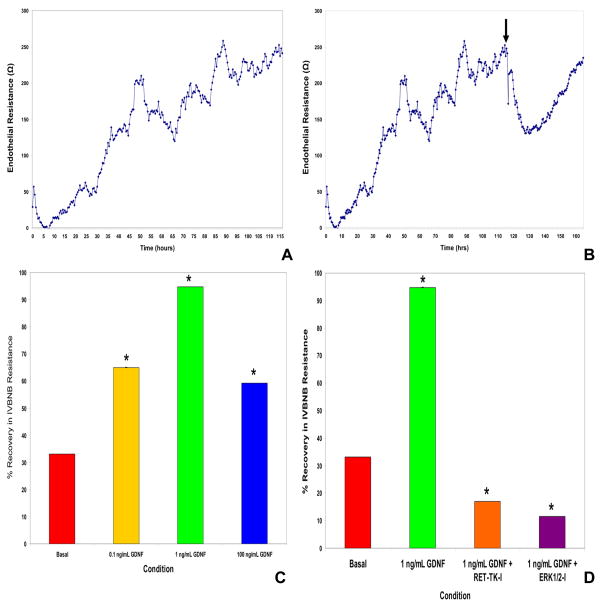
Figure 4. GDNF and its signaling pathways in the recovery of human blood-nerve barrier resistance in vitro following serum withdrawal.
Using continuous electrical cell impedance sensing, primary human endoneurial endothelial cells cultured on glutaraldehyde-crosslinked rat tail collagen-coated wells achieve steady transendothelial electrical resistance 5 days after plating (A). Following serum withdrawal from confluent cultures (B) with concomitant inclusion of 1 ng/mL GDNF (black arrow), there is a rapid drop in resistance for about 18 hours followed by a gradual improvement in resistance over the next 30 hours. This response with the in vitro blood-nerve barrier (IVBNB) is dose-dependent (C), and maximal with 1 ng/mL GDNF, as observed in our published study using transwell inserts. GDNF-mediated recovery in IVBNB resistance is dependent on RET-tyrosine kinase (RET-TK) signaling with evidence supporting downstream involvement of the mitogen activated protein kinase pathway, demonstrated by inhibition with specific cell-permeable inhibitors against RET-TK and ERK1/2 (D), respectively. * indicates p<0.05 for these preliminary experiments.
Vascular endothelial growth factor (VEGF) has been implicated as an important angiogenic and neurotrophic factor in peripheral nerve regeneration following injury in rodent models.[59, 60, 62, 63] We are currently evaluating the role of VEGF and other growth factors such as basic fibroblast growth factor in human blood-nerve barrier endothelial cell proliferation, angiogenesis and wound healing in vitro. In support of data from rodent models, our ongoing unpublished work demonstrates an important role for exogenous VEGF in the above processes required for vascular repair and remodeling after injury (Figure 5). Interestingly, there is evidence that Schwann cells secrete VEGF during peripheral nerve repair following chronic compression.[63] Our data imply important roles for both VEGF and GDNF at different stages of neurovascular repair in peripheral nerves, providing molecular targets with translational potential in human peripheral neuropathies, as demonstrated by a recently concluded phase I clinical trial of VEGF gene therapy in diabetic neuropathy.[75]
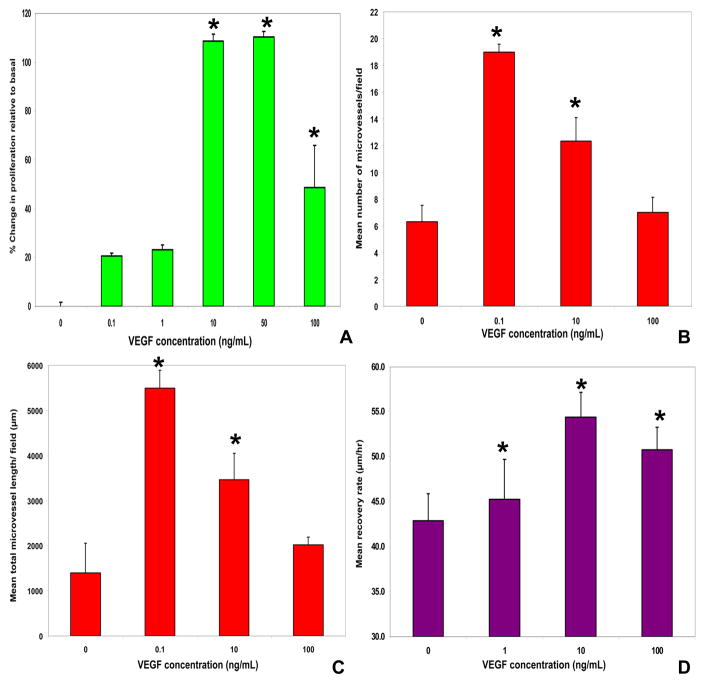
Figure 5. VEGF effects on human blood-nerve barrier endothelial cell proliferation, angiogenesis and wound healing in vitro.
The dose-dependent effects of VEGF165 in primary endoneurial endothelial cell proliferation relative to basal proliferation without added mitogens using the non-radioactive WST-1 assay is shown, with maximal proliferation observed with 10 and 50 ng/mL (A). Using a 4hr Matrigel® angiogenesis assay, 0.1 ng/mL VEGF165 maximally increased the mean number of microvessels (B) and their mean total length (C) per field (1700 μm × 1270 μm) in a dose-dependent manner relative to basal medium without added mitogens. A sterile micropipette wound healing assay performed on confluent primary endoneurial endothelial cell cultures demonstrates a dose-dependent rate of recovery (based on endothelial cell migration across the deficits between 4–18 hours after the injury), maximal with 10 ng/mL VEGF (D). Interestingly, 10 ng/mL VEGF165 induced complete wound healing between 18–30 hours after injury by endothelial cell proliferation. The VEGF-mediated effects on proliferation, angiogenesis and wound healing are significantly enhanced by heparin (data not shown). * indicates p<0.05 for these preliminary experiments.
Conclusions
Scientific endeavors in the Neuromuscular Immunopathology Research Laboratory at Baylor College of Medicine are geared towards developing targeted molecular therapies for immune-mediated acute and chronic peripheral neuroinflammatory diseases, GBS and CIDP, as well as for peripheral neuropathies associated with endoneurial microvascular compromise. These translational efforts are guided by human observational studies, and utilize in vitro and in vivo models that take into consideration the importance of the blood-nerve barrier and its interactions with hematogenous leukocytes or exogenous molecules in disease pathogenesis and potential treatment. Despite the limitations of using in-bred animals to model human peripheral neuroinflammation, pre-clinical proof-of-principle therapeutic studies in murine models are designed to take into account the disease states of untreated patients in clinical practice and assessed using realistic outcome measures applicable to clinical trials. In vitro studies using untreated affected patient leukocytes aim to mimic early hematogenous leukocyte-endothelial cell interactions that may be necessary for peripheral nerve inflammation, recognizing the potential difficulties inherent to these models. Modeling endoneurial microvascular injury provides some insight into how the blood-nerve barrier may adapt and respond to extrinsic insult. Future translational paradigms for axonal regeneration would need to consider drug permeability characteristics at the blood-nerve barrier early on in the drug discovery process. Our ultimate goal is to aid with the discovery and direct translation of targeted, efficacious molecular therapies for peripheral neuroinflammation and peripheral neuropathies.
Acknowledgments
Work in the Neuromuscular Immunopathology Research Laboratory is supported by the National Institute of Neurological Disorders and Stroke of the National Institutes of Health grants R21NS073702, R21NS078226 and R01NS075212. The content is solely the responsibility of the author and does not necessarily represent the official views of the National Institutes of Health. Special thanks to past and current members of the laboratory and collaborators who have assisted with the development and characterization of these model systems and experimental assays.
References
- 1.Bennett G. Neuropathic pain: new insights, new interventions. Hosp Pract (Minneap) 1998;33:95–98. 101–104, 107–110. doi: 10.3810/hp.1998.10.114. passim. [DOI] [PubMed] [Google Scholar]
- 2.Moalem G, Tracey D. Immune and inflammatory mechanisms in neuropathic pain. Brain Res Rev. 2006;51:240–264. doi: 10.1016/j.brainresrev.2005.11.004. [DOI] [PubMed] [Google Scholar]
- 3.White FA, Jung H, Miller RJ. Chemokines and the pathophysiology of neuropathic pain. Proc Natl Acad Sci U S A. 2007;104:20151–20158. doi: 10.1073/pnas.0709250104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Dworkin R. An overview of neuropathic pain: syndromes, symptoms, signs, and several mechanisms. Clin J Pain. 2002;18:343–349. doi: 10.1097/00002508-200211000-00001. [DOI] [PubMed] [Google Scholar]
- 5.Kieseier B, Kiefer R, Gold R, Hemmer B, Willison H, Hartung H. Advances in understanding and treatment of immune-mediated disorders of the peripheral nervous system. Muscle Nerve. 2004;30:131–156. doi: 10.1002/mus.20076. [DOI] [PubMed] [Google Scholar]
- 6.Meyerzu Hörste G, Hartung H, Kieseier B. From bench to bedside--experimental rationale for immune-specific therapies in the inflamed peripheral nerve. Nat Clin Pract Neurol. 2007;3:198–211. doi: 10.1038/ncpneuro0452. [DOI] [PubMed] [Google Scholar]
- 7.McCarberg BH, Billington R. Consequences of neuropathic pain: quality-of-life issues and associated costs. Am J Manag Care. 2006;12:S263–268. [PubMed] [Google Scholar]
- 8.Hartung H, Willison H, Kieseier B. Acute immunoinflammatory neuropathy: update on Guillain-Barré syndrome. Curr Opin Neurol. 2002;15:571–577. doi: 10.1097/00019052-200210000-00008. [DOI] [PubMed] [Google Scholar]
- 9.Hughes R, Cornblath D. Guillain-Barré syndrome. Lancet. 2005;366:1653–1666. doi: 10.1016/S0140-6736(05)67665-9. [DOI] [PubMed] [Google Scholar]
- 10.Kieseier B, Kiefer R, Gold R, Hemmer B, Willison H, Hartung H. Advances in understanding and treatment of immune-mediated disorders of the peripheral nervous system. Muscle Nerve. 2004;30:131–156. doi: 10.1002/mus.20076. [DOI] [PubMed] [Google Scholar]
- 11.Teitelbaum JS, Borel CO. Respiratory dysfunction in Guillain-Barre syndrome. Clin Chest Med. 1994;15:705–714. [PubMed] [Google Scholar]
- 12.Orlikowski D, Prigent H, Sharshar T, Lofaso F, Raphael JC. Respiratory dysfunction in Guillain-Barre Syndrome. Neurocrit Care. 2004;1:415–422. doi: 10.1385/NCC:1:4:415. [DOI] [PubMed] [Google Scholar]
- 13.Frenzen P. Economic cost of Guillain-Barré syndrome in the United States. Neurology. 2008;71:21–27. doi: 10.1212/01.wnl.0000316393.54258.d1. [DOI] [PubMed] [Google Scholar]
- 14.Meyerzu Hörste G, Hartung H, Kieseier B. From bench to bedside--experimental rationale for immune-specific therapies in the inflamed peripheral nerve. Nat Clin Pract Neurol. 2007;3:198–211. doi: 10.1038/ncpneuro0452. [DOI] [PubMed] [Google Scholar]
- 15.Kieseier B, Dalakas M, Hartung H. Immune mechanisms in chronic inflammatory demyelinating neuropathy. Neurology. 2002;59:S7–12. doi: 10.1212/wnl.59.12_suppl_6.s7. [DOI] [PubMed] [Google Scholar]
- 16.Chia L, Fernandez A, Lacroix C, Adams D, Planté V, Said G. Contribution of nerve biopsy findings to the diagnosis of disabling neuropathy in the elderly. A retrospective review of 100 consecutive patients. Brain. 1996;119(Pt 4):1091–1098. doi: 10.1093/brain/119.4.1091. [DOI] [PubMed] [Google Scholar]
- 17.Bouchard C, Lacroix C, Planté V, Adams D, Chedru F, Guglielmi J, et al. Clinicopathologic findings and prognosis of chronic inflammatory demyelinating polyneuropathy. Neurology. 1999;52:498–503. doi: 10.1212/wnl.52.3.498. [DOI] [PubMed] [Google Scholar]
- 18.Rizzuto N, Morbin M, Cavallaro T, Ferrari S, Fallahi M, Galiazzo Rizzuto S. Focal lesions area feature of chronic inflammatory demyelinating polyneuropathy (CIDP) Acta Neuropathol. 1998;96:603–609. doi: 10.1007/s004010050941. [DOI] [PubMed] [Google Scholar]
- 19.Reina M, López A, Villanueva M, de Andrés J, León G. Morphology of peripheral nerves, their sheaths, and their vascularization. Rev Esp Anestesiol Reanim. 2000;47:464–475. [PubMed] [Google Scholar]
- 20.Reina M, López A, Villanueva M, De Andrés J, Machés F. The blood-nerve barrier in peripheral nerves. Rev Esp Anestesiol Reanim. 2003;50:80–86. [PubMed] [Google Scholar]
- 21.Olsson Y. Studies on vascular permeability in peripheral nerves. I. Distribution of circulating fluorescent serum albumin in normal, crushed and sectioned rat sciatic nerve. Acta Neuropathol. 1966;7:1–15. doi: 10.1007/BF00686605. [DOI] [PubMed] [Google Scholar]
- 22.Olsson Y. Topographical differences in the vascular permeability of the peripheral nervous system. Acta Neuropathol. 1968;10:26–33. doi: 10.1007/BF00690507. [DOI] [PubMed] [Google Scholar]
- 23.Olsson Y. Microenvironment of the peripheral nervous system under normal and pathological conditions. Crit Rev Neurobiol. 1990;5:265–311. [PubMed] [Google Scholar]
- 24.Olsson Y. Studies on vascular permeability in peripheral nerves. IV. Distribution of intravenously injected protein tracers in the peripheral nervous system of various species. Acta Neuropathol. 1971;17:114–126. doi: 10.1007/BF00687487. [DOI] [PubMed] [Google Scholar]
- 25.Hultström D, Malmgren L, Gilstring D, Olsson Y. FITC-Dextrans as tracers for macromolecular movements in the nervous system. A freeze-drying method for dextrans of various molecular sizes injected into normal animals. Acta Neuropathol. 1983;59:53–62. doi: 10.1007/BF00690317. [DOI] [PubMed] [Google Scholar]
- 26.Malmgren L, Olsson Y. Differences between the peripheral and the central nervous system in permeability to sodium fluorescein. J Comp Neurol. 1980;191:103–107. doi: 10.1002/cne.901910106. [DOI] [PubMed] [Google Scholar]
- 27.Poduslo J, Curran G, Berg C. Macromolecular permeability across the blood-nerve and blood-brain barriers. Proc Natl Acad Sci U S A. 1994;91:5705–5709. doi: 10.1073/pnas.91.12.5705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Huebner E, Strittmatter S. Axon regeneration in the peripheral and central nervous systems. Results Probl Cell Differ. 2009;48:339–351. doi: 10.1007/400_2009_19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Scherer S. The biology and pathobiology of Schwann cells. Curr Opin Neurol. 1997;10:386–397. doi: 10.1097/00019052-199710000-00006. [DOI] [PubMed] [Google Scholar]
- 30.Höke A, Mi R. In search of novel treatments for peripheral neuropathies and nerve regeneration. Discov Med. 2007;7:109–112. [PubMed] [Google Scholar]
- 31.Chen Z, Yu W, Strickland S. Peripheral regeneration. Annu Rev Neurosci. 2007;30:209–233. doi: 10.1146/annurev.neuro.30.051606.094337. [DOI] [PubMed] [Google Scholar]
- 32.Poduslo J, Curran G, Dyck P. Increase in albumin, IgG, and IgM blood-nerve barrier indices in human diabetic neuropathy. Proc Natl Acad Sci U S A. 1988;85:4879–4883. doi: 10.1073/pnas.85.13.4879. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Giannini C, Dyck P. Ultrastructural morphometric abnormalities of sural nerve endoneurial microvessels in diabetes mellitus. Ann Neurol. 1994;36:408–415. doi: 10.1002/ana.410360312. [DOI] [PubMed] [Google Scholar]
- 34.Malik R, Newrick P, Sharma A, Jennings A, Ah-See A, Mayhew T, et al. Microangiopathy in human diabetic neuropathy: relationship between capillary abnormalities and the severity of neuropathy. Diabetologia. 1989;32:92–102. doi: 10.1007/BF00505180. [DOI] [PubMed] [Google Scholar]
- 35.Malik R, Veves A, Masson E, Sharma A, Ah-See A, Schady W, et al. Endoneurial capillary abnormalities in mild human diabetic neuropathy. J Neurol Neurosurg Psychiatry. 1992;55:557–561. doi: 10.1136/jnnp.55.7.557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Ubogu EE, Yosef N, Xia RH, Sheikh KA. Behavioral, electrophysiological, and histopathological characterization of a severe murine chronic demyelinating polyneuritis model. J Peripher Nerv Syst. 2012;17:53–61. doi: 10.1111/j.1529-8027.2012.00375.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Xia R, Yosef N, Ubogu E. Clinical, electrophysiological and pathologic correlations in a severe murine experimental autoimmune neuritis model of Guillain-Barré syndrome. J Neuroimmunol. 2010;219:54–63. doi: 10.1016/j.jneuroim.2009.11.019. [DOI] [PubMed] [Google Scholar]
- 38.Yosef N, Xia R, Ubogu E. Development and characterization of a novel human in vitro blood-nerve barrier model using primary endoneurial endothelial cells. J Neuropathol Exp Neurol. 2010;69:82–97. doi: 10.1097/NEN.0b013e3181c84a9a. [DOI] [PubMed] [Google Scholar]
- 39.Calida D, Kremlev S, Fujioka T, Hilliard B, Ventura E, Constantinescu C, et al. Experimental allergic neuritis in the SJL/J mouse: induction of severe and reproducible disease with bovine peripheral nerve myelin and pertussis toxin with or without interleukin-12. J Neuroimmunol. 2000;107:1–7. doi: 10.1016/s0165-5728(00)00249-6. [DOI] [PubMed] [Google Scholar]
- 40.Kieseier B, Tani M, Mahad D, Oka N, Ho T, Woodroofe N, et al. Chemokines and chemokine receptors in inflammatory demyelinating neuropathies: a central role for IP-10. Brain. 2002;125:823–834. doi: 10.1093/brain/awf070. [DOI] [PubMed] [Google Scholar]
- 41.Lu MO, Zhu J. The role of cytokines in Guillain-Barré syndrome. J Neurol. 2010 doi: 10.1007/s00415-010-5836-5. [DOI] [PubMed] [Google Scholar]
- 42.Orlikowski D, Chazaud B, Plonquet A, Poron F, Sharshar T, Maison P, et al. Monocyte chemoattractant protein 1 and chemokine receptor CCR2 productions in Guillain-Barré syndrome and experimental autoimmune neuritis. J Neuroimmunol. 2003;134:118–127. doi: 10.1016/s0165-5728(02)00393-4. [DOI] [PubMed] [Google Scholar]
- 43.Press R, Pashenkov M, Jin J, Link H. Aberrated levels of cerebrospinal fluid chemokines in Guillain-Barré syndrome and chronic inflammatory demyelinating polyradiculoneuropathy. J Clin Immunol. 2003;23:259–267. doi: 10.1023/a:1024532715775. [DOI] [PubMed] [Google Scholar]
- 44.Xia RH, Yosef N, Ubogu EE. Selective expression and cellular localization of pro-inflammatory chemokine ligand/receptor pairs in the sciatic nerves of a severe murine experimental autoimmune neuritis model of Guillain-Barré syndrome. Neuropathol Appl Neurobiol. 2010;36:388–398. doi: 10.1111/j.1365-2990.2010.01092.x. [DOI] [PubMed] [Google Scholar]
- 45.Ubogu E, Cossoy M, Ransohoff R. The expression and function of chemokines involved in CNS inflammation. Trends Pharmacol Sci. 2006;27:48–55. doi: 10.1016/j.tips.2005.11.002. [DOI] [PubMed] [Google Scholar]
- 46.Ubogu EE. Chemokine receptors as specific anti-inflammatory targets in peripheral nerves. Endocr Metab Immune Disord Drug Targets. 2011;11:141–153. doi: 10.2174/187153011795564124. [DOI] [PubMed] [Google Scholar]
- 47.Alon R, Ley K. Cells on the run: shear-regulated integrin activation in leukocyte rolling and arrest on endothelial cells. Curr Opin Cell Biol. 2008;20:525–532. doi: 10.1016/j.ceb.2008.04.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Man S, Ubogu E, Ransohoff R. Inflammatory cell migration into the central nervous system: a few new twists on an old tale. Brain Pathol. 2007;17:243–250. doi: 10.1111/j.1750-3639.2007.00067.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Yosef N, Ubogu EE. alpha(M) beta(2) -integrin-intercellular adhesion molecule-1 interactions drive the flow-dependent trafficking of Guillain-Barre syndrome patient derived mononuclear leukocytes at the blood-nerve barrier in vitro. J Cell Physiol. 2012;227:3857–3875. doi: 10.1002/jcp.24100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Silverman M, Zamora D, Pan Y, Texeira P, Planck S, Rosenbaum J. Cell adhesion molecule expression in cultured human iris endothelial cells. Invest Ophthalmol Vis Sci. 2001;42:2861–2866. [PubMed] [Google Scholar]
- 51.Bö L, Peterson J, M⊘rk S, Hoffman P, Gallatin W, Ransohoff R, et al. Distribution of immunoglobulin superfamily members ICAM-1, -2, -3, and the beta 2 integrin LFA-1 in multiple sclerosis lesions. J Neuropathol Exp Neurol. 1996;55:1060–1072. [PubMed] [Google Scholar]
- 52.Ubogu E, Callahan M, Tucky B, Ransohoff R. Determinants of CCL5-driven mononuclear cell migration across the blood-brain barrier. Implications for therapeutically modulating neuroinflammation. J Neuroimmunol. 2006;179:132–144. doi: 10.1016/j.jneuroim.2006.06.004. [DOI] [PubMed] [Google Scholar]
- 53.Salomon B, Rhee L, Bour-Jordan H, Hsin H, Montag A, Soliven B, et al. Development of spontaneous autoimmune peripheral polyneuropathy in B7-2-deficient NOD mice. J Exp Med. 2001;194:677–684. doi: 10.1084/jem.194.5.677. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Louvet C, Kabre B, Davini D, Martinier N, Su M, DeVoss J, et al. A novel myelin P0-specific T cell receptor transgenic mouse develops a fulminant autoimmune peripheral neuropathy. J Exp Med. 2009;206:507–514. doi: 10.1084/jem.20082113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Nagamatsu M, Terao S, Misu K, Li M, Hattori N, Ichimura M, et al. Axonal and perikaryal involvement in chronic inflammatory demyelinating polyneuropathy. J Neurol Neurosurg Psychiatry. 1999;66:727–733. doi: 10.1136/jnnp.66.6.727. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Lindenlaub T, Sommer C. Cytokines in sural nerve biopsies from inflammatory and non-inflammatory neuropathies. Acta Neuropathol. 2003;105:593–602. doi: 10.1007/s00401-003-0689-y. [DOI] [PubMed] [Google Scholar]
- 57.Putzu GA, Figarella-Branger D, Bouvier-Labit C, Liprandi A, Bianco N, Pellissier JF. Immunohistochemical localization of cytokines, C5b-9 and ICAM-1 in peripheral nerve of Guillain-Barré syndrome. J Neurol Sci. 2000;174:16–21. doi: 10.1016/s0022-510x(99)00328-7. [DOI] [PubMed] [Google Scholar]
- 58.Nishimura N, Schaffer C, Friedman B, Tsai P, Lyden P, Kleinfeld D. Targeted insult to subsurface cortical blood vessels using ultrashort laser pulses: three models of stroke. Nat Methods. 2006;3:99–108. doi: 10.1038/nmeth844. [DOI] [PubMed] [Google Scholar]
- 59.Pereira Lopes FR, Lisboa BC, Frattini F, Almeida FM, Tomaz MA, Matsumoto PK, et al. Enhancement of sciatic nerve regeneration after vascular endothelial growth factor (VEGF) gene therapy. Neuropathol Appl Neurobiol. 2011;37:600–612. doi: 10.1111/j.1365-2990.2011.01159.x. [DOI] [PubMed] [Google Scholar]
- 60.Pola R, Aprahamian TR, Bosch-Marce M, Curry C, Gaetani E, Flex A, et al. Age-dependent VEGF expression and intraneural neovascularization during regeneration of peripheral nerves. Neurobiol Aging. 2004;25:1361–1368. doi: 10.1016/j.neurobiolaging.2004.02.028. [DOI] [PubMed] [Google Scholar]
- 61.Sun Y, Jin K, Xie L, Childs J, Mao X, Logvinova A, et al. VEGF-induced neuroprotection, neurogenesis, and angiogenesis after focal cerebral ischemia. J Clin Invest. 2003;111:1843–1851. doi: 10.1172/JCI17977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Zachary I. Neuroprotective role of vascular endothelial growth factor: signalling mechanisms, biological function, and therapeutic potential. Neurosignals. 2005;14:207–221. doi: 10.1159/000088637. [DOI] [PubMed] [Google Scholar]
- 63.Gupta R, Gray M, Chao T, Bear D, Modafferi E, Mozaffar T. Schwann cells upregulate vascular endothelial growth factor secondary to chronic nerve compression injury. Muscle Nerve. 2005;31:452–460. doi: 10.1002/mus.20272. [DOI] [PubMed] [Google Scholar]
- 64.Yosef N, Ubogu EE. GDNF restores human blood-nerve barrier function via RET tyrosine kinase-mediated cytoskeletal reorganization. Microvasc Res. 2012;83:298–310. doi: 10.1016/j.mvr.2012.01.005. [DOI] [PubMed] [Google Scholar]
- 65.Shen W, Li S, Chung SH, Zhu L, Stayt J, Su T, et al. Tyrosine phosphorylation of VE-cadherin and claudin-5 is associated with TGF-beta1-induced permeability of centrally derived vascular endothelium. Eur J Cell Biol. 2011;90:323–332. doi: 10.1016/j.ejcb.2010.10.013. [DOI] [PubMed] [Google Scholar]
- 66.Bär KJ, Saldanha GJ, Kennedy AJ, Facer P, Birch R, Carlstedt T, et al. GDNF and its receptor component Ret in injured human nerves and dorsal root ganglia. Neuroreport. 1998;9:43–47. doi: 10.1097/00001756-199801050-00009. [DOI] [PubMed] [Google Scholar]
- 67.Beck KD, Valverde J, Alexi T, Poulsen K, Moffat B, Vandlen RA, et al. Mesencephalic dopaminergic neurons protected by GDNF from axotomy-induced degeneration in the adult brain. Nature. 1995;373:339–341. doi: 10.1038/373339a0. [DOI] [PubMed] [Google Scholar]
- 68.Bennett DL, Michael GJ, Ramachandran N, Munson JB, Averill S, Yan Q, et al. A distinct subgroup of small DRG cells express GDNF receptor components and GDNF is protective for these neurons after nerve injury. J Neurosci. 1998;18:3059–3072. doi: 10.1523/JNEUROSCI.18-08-03059.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Boyd JG, Gordon T. Glial cell line-derived neurotrophic factor and brain-derived neurotrophic factor sustain the axonal regeneration of chronically axotomized motoneurons in vivo. Exp Neurol. 2003;183:610–619. doi: 10.1016/s0014-4886(03)00183-3. [DOI] [PubMed] [Google Scholar]
- 70.Hammarberg H, Piehl F, Cullheim S, Fjell J, Hökfelt T, Fried K. GDNF mRNA in Schwann cells and DRG satellite cells after chronic sciatic nerve injury. Neuroreport. 1996;7:857–860. doi: 10.1097/00001756-199603220-00004. [DOI] [PubMed] [Google Scholar]
- 71.Henderson CE, Phillips HS, Pollock RA, Davies AM, Lemeulle C, Armanini M, et al. GDNF: a potent survival factor for motoneurons present in peripheral nerve and muscle. Science. 1994;266:1062–1064. doi: 10.1126/science.7973664. [DOI] [PubMed] [Google Scholar]
- 72.Pascual A, Hidalgo-Figueroa M, Piruat JI, Pintado CO, Gomez-Diaz R, Lopez-Barneo J. Absolute requirement of GDNF for adult catecholaminergic neuron survival. Nat Neurosci. 2008;11:755–761. doi: 10.1038/nn.2136. [DOI] [PubMed] [Google Scholar]
- 73.Shi JY, Liu GS, Liu LF, Kuo SM, Ton CH, Wen ZH, et al. Glial cell line-derived neurotrophic factor gene transfer exerts protective effect on axons in sciatic nerve following constriction-induced peripheral nerve injury. Hum Gene Ther. 2011;22:721–731. doi: 10.1089/hum.2010.036. [DOI] [PubMed] [Google Scholar]
- 74.Springer JE, Seeburger JL, He J, Gabrea A, Blankenhorn EP, Bergman LW. cDNA sequence and differential mRNA regulation of two forms of glial cell line-derived neurotrophic factor in Schwann cells and rat skeletal muscle. Exp Neurol. 1995;131:47–52. doi: 10.1016/0014-4886(95)90006-3. [DOI] [PubMed] [Google Scholar]
- 75.Ropper AH, Gorson KC, Gooch CL, Weinberg DH, Pieczek A, Ware JH, et al. Vascular endothelial growth factor gene transfer for diabetic polyneuropathy: a randomized, double-blinded trial. Ann Neurol. 2009;65:386–393. doi: 10.1002/ana.21675. [DOI] [PMC free article] [PubMed] [Google Scholar]