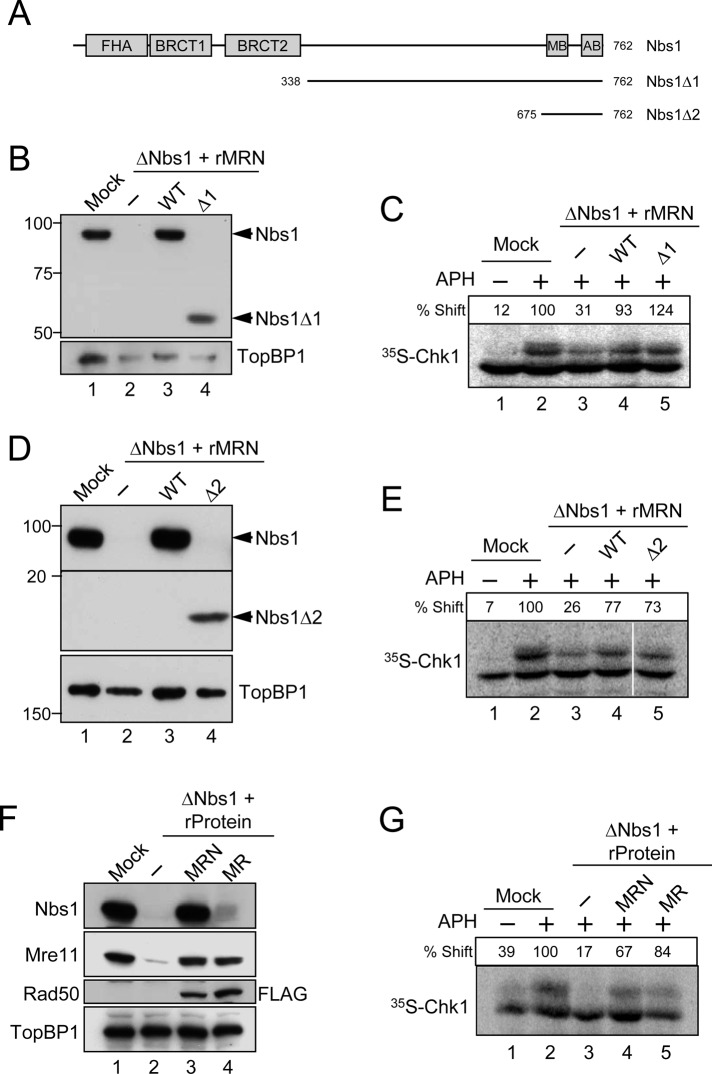
FIGURE 4:
The Nbs1 component of the MRN complex is dispensable for the APH-induced activation of Chk1. (A) Domains of the Xenopus Nbs1 protein. The protein contains an FHA domain and two BRCT domains in its N-terminal region and an Mre11-binding domain (MB) and ATM-binding domain (AB) in its C-terminal region. Two deletion constructs of Nbs1 (∆1 and ∆2) are depicted. (B and D) Egg extracts were mock-treated with control antibodies (lane 1) or immunodepleted with anti-Nbs1 antibodies (lanes 2–4). Extracts were supplemented with control buffer (B and D, lanes 1 and 2), wild-type rMRN complex (B and D, lane 3), mutant rMRN∆1 complex (B, lane 4), or mutant rMRN∆2 complex (D, lane 4). Extracts were immunoblotted with anti-Nbs1 and anti-TopBP1 antibodies. (C and E) Extracts from (B) and (D), respectively, were incubated with [35S]Chk1 in the absence (lane 1) or presence (lanes 2–5) of APH. Nuclear fractions from the extracts were processed for phosphorimaging to assess phosphorylation-dependent shifting of Chk1. Numbers above each lane denote quantitation of phosphorylation relative to mock-depleted, APH-treated extracts. (F) Mock-treated (lane 1) or Nbs1-depleted extracts (lanes 2–4) were supplemented with control buffer (lanes 1 and 2), wild-type rMRN complex (lane 3), or recombinant MR complex lacking Nbs1 (lane 4). For these experiments, the Rad50 subunit in the trimeric MRN and dimeric MR complexes contained a C-terminal FLAG tag (see Materials and Methods). Extracts were immunoblotted for the indicated proteins. (G) Extracts in (F) were incubated with [35S]Chk1 in the absence (lane 1) or presence (lanes 2–5) of APH. Nuclear fractions from the extracts were processed for phosphorimaging.