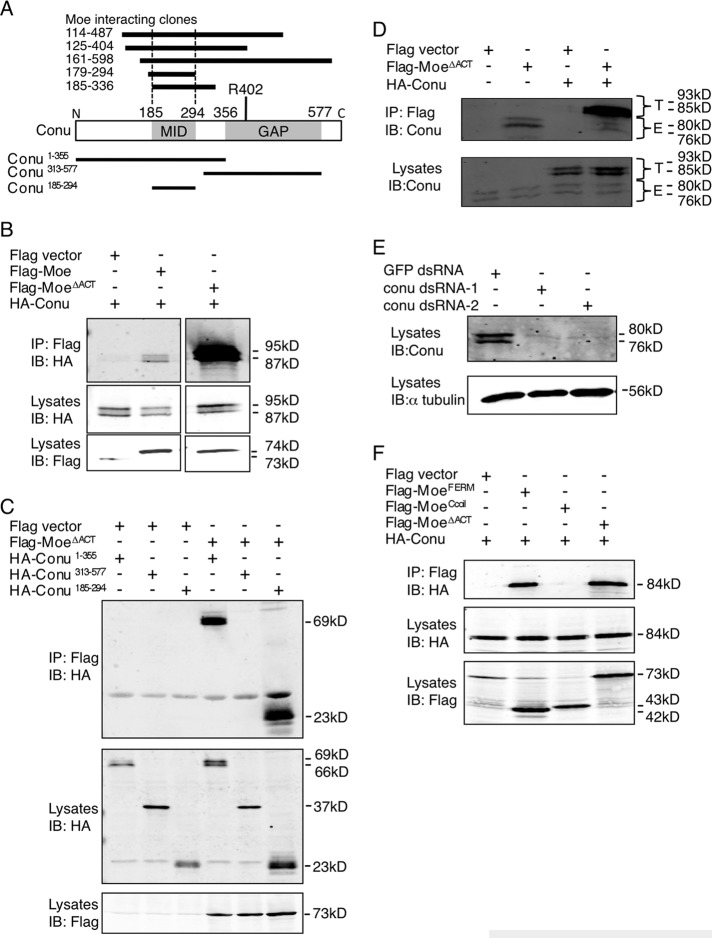
FIGURE 1:
Conu forms a complex with Moe. (A) Schematic diagram of Conu's structure with amino acid coordinates indicated above the diagram. The Moe interaction domain (MID) and the GAP domain with a predicted arginine finger required for GAP activity at amino acid 402, are indicated. The MID is defined by the overlap of five unique Conu clones that interacted with Moe in the yeast two-hybrid screen, as indicated above the Conu diagram. Conu fragments used for co-IP experiments with Moe are indicated below the schematic. (B–F) S2 cells were cotransfected with expression constructs for the indicated forms of Moe and Conu. (B) Conu coimmunoprecipitates with wild-type Moe, and more strongly with a form of Moe lacking the F-actin binding domain (ΔACT). (C) Moe∆ACT can coimmunoprecipitate N-terminal fragments of Conu (Conu 1–355 and Conu 185–294) that contain the MID, but not the C-terminal GAP domain of Conu (aa 313–577). (D) Endogenous Conu protein (E, endogenous) in S2 cells can be coimmunoprecipitated with Moe∆ACT. Epitope-tagged Conu was used as a positive control (T, tagged). (E) Specificity of the Conu antibody is demonstrated by dsRNA-mediated knockdown against two nonoverlapping regions of Conu. GFP dsRNA serves as a negative control. (F) The Moe FERM domain, but not the coiled-coil region, is sufficient to coimmunoprecipitate Conu.