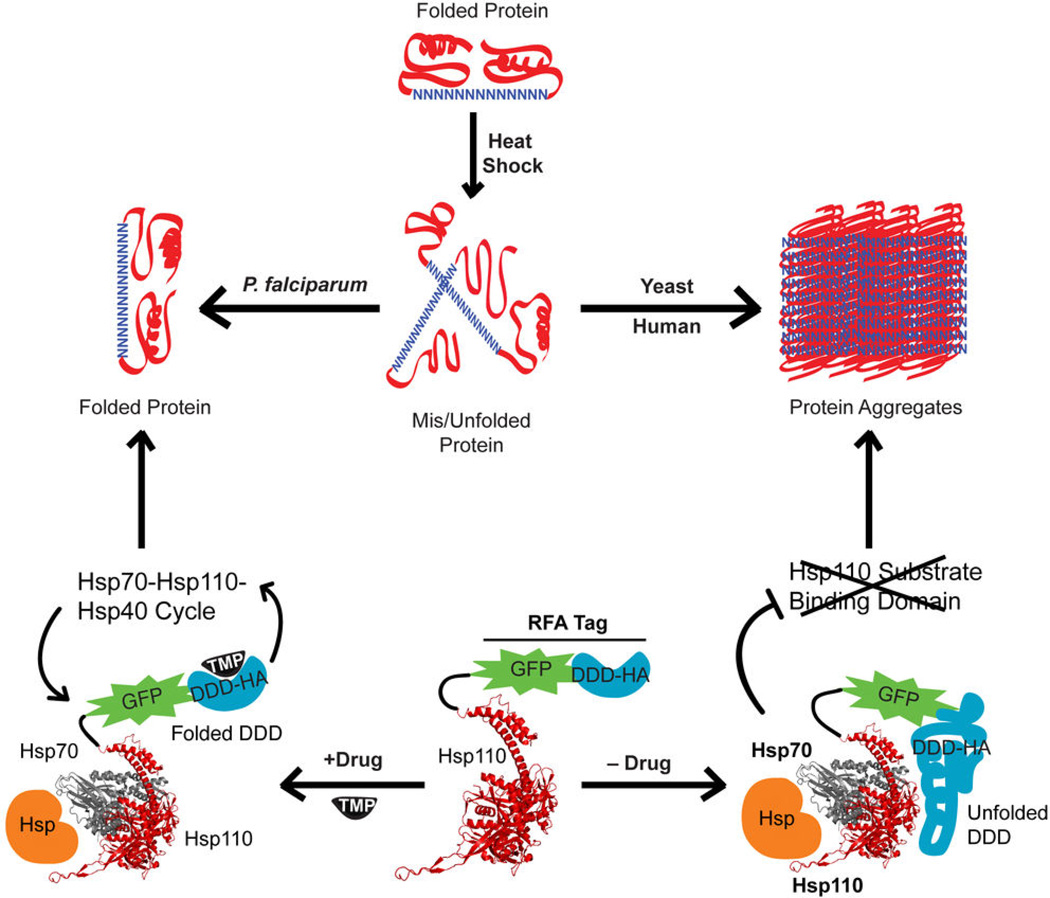
Figure 7.
Proposed role of Hsp110 in maintaining an aggregate-free Asn repeat-rich parasite proteome. Proteins containing Asn-rich regions are misfolded or unfolded during heat shock. The misfolded proteins form aggregates in human and yeast cells. However, in the malaria parasite, despite an abundance of Asn repeat-rich proteins, there is no protein aggregation. The prevention of protein aggregation in P. falciparum depends upon the activity of PfHsp110c that acts in a network with other chaperones (Hsp70 and other Hsp). PfHsp110c-RFA function is dependent upon the stabilizing ligand, TMP. When TMP is present, Hsp110 can productively function in the Hsp70-Hsp110-Hsp40 cycle resulting in a stable Asn repeat-rich proteome. In the absence of TMP, the DDD within the RFA tag is unfolded. The unfolded DDD fused to Hsp110 results in the inhibition of chaperone activity. This leads to protein aggregation in the Asn repeat-rich proteome. The Hsp110 and Hsp70 structures (PDB: 3C7N) were rendered using PyMol.