Abstract
Change in oceanographic conditions causes structural alterations in marine fish communities, but this effect may go undetected as most monitoring programs until recently mainly have focused on oceanography and commercial species rather than on whole ecosystems. In this paper, the objective is to describe the spatial and temporal changes in the Barents Sea fish community in the period 1992–2004 while taking into consideration the observed abundance and biodiversity patterns for all 82 observed fish species. We found that the spatial structure of the Barents Sea fish community was determined by abiotic factors such as temperature and depth. The observed species clustered into a deep assemblage, a warm water southern assemblage, both associated with Atlantic water, and a cold water north-eastern assemblage associated with mixed water. The latitude of the cold water NE and warm water S assemblages varied from year to year, but no obvious northward migration was observed over time. In the period 1996–1999 we observed a significant reduction in total fish biomass, abundance, mean fish weight, and a change in community structure including an increase in the pelagic/demersal ratio. This change in community structure is probably due to extremely cold conditions in 1996 impacting on a fish community exposed to historically high fishing rates. After 1999 the fish community variables such as biomass, abundance, mean weight, P/D ratio as well as community composition did not return to levels of the early 90s, although fishing pressure and climatic conditions returned to earlier levels.
Introduction
The Barents Sea ecosystem has been considered ecologically ‘healthy’ [1], [2] and many of the commercial fish stocks, especially the Northeast Arctic cod (Gadus morhua), are in good shape [3]. However, due to rapid climate change in the Arctic, where local temperature increase is expected to be twice the global average [4], [5], major changes in the marine ecosystems are expected. In the adjacent North Sea, changes in large-scale hydro-meteorological forcing has caused a change in individual species and key ecosystem parameters, such as diversity from phytoplankton to fish [6]. Local temperature increase in the Barents Sea is expected to lead to migration of Atlantic fish species northwards [5], [7], [8], but more complex and structural community changes may also occur [9]–[11].
The Barents Sea has been studied for several decades, and approximately 500 survey days are spent in the vast sea area annually. Change in climatic conditions can cause structural alterations in marine fish communities, but this effect may go undetected as the monitoring programs in the Barents Sea have, until 2004 [12], mainly focused on oceanography and commercial species (e.g. 0-group surveys on commercial juveniles, shrimp surveys and gadoid fish surveys) rather than on the whole ecosystem. Single species responses may not give a good indication of possible changes in the ecosystem due to the large inter-annual variability of single stocks, and because fishing may conceal climate change effects [13]. In general, change in climatic conditions and fishing have been the most important drivers for structural change in marine ecosystems [14], for instance as seen in the previously cod-dominated community in the NW-Atlantic [15].
A community change may consist of a change in structural properties, such as species composition or change in functional roles. Changes in species composition can be indicative of ecological regime shifts [16], [17]. The evidence for such shifts in the oceans, for instance a persistent change in biomass or structural changes over several trophic levels, has been documented for the North Pacific [18], [19], the Northwest Atlantic [15], and the North Sea [6]. Such ecological changes are often related to shifts in oceanographic conditions and overfishing [10], and may be difficult to reverse. Regime shifts have only been detected through retrospective analysis [20], [21], for instance through benthic-pelagic decoupling in Northwest Atlantic fish communities resulting in increased pelagic fish abundance and biomass [22]–[24]. Pelagic species change abundance and distributions more rapidly than demersal species, due to faster lifecycles and smaller body sizes [25], [26], while both may be subject to fishing. The pelagic/demersal (P/D) ratio of the fish community is therefore a suitable descriptor of temporal changes in an ecosystem.
When investigating community change, the baseline community structure needs to be known. For the Barents Sea, the fish fauna and the biogeographical distribution patterns of species are well known from extensive taxonomical studies [27]. Fish community studies, i.e. descriptions of assemblages of species inhabiting specific habitats or sub-areas of the Barents Sea, are limited in geographical scope and time 28–34. These studies show that assemblages can be identified in subareas, but they do not provide information on temporal variability.
The main objective of this study is to document the temporal and spatial changes in the Barents Sea fish assemblages in relation to environmental parameters. Fish species that are not targeted by fisheries are included in the analysis, providing valuable, additional ecological information on structural changes [35], [36]. Spatial and temporal changes in the Barents Sea fish communities are investigated by studying fish biomass, abundance, mean weight, diversity, P/D ratio and species composition in relation to driving forces, such as bottom temperature, the North Atlantic Oscillation (NAO) index and fishing (demersal landings).
Materials and Methods
Ethics Statement
The surveys were conducted by marine research institutes financed by the Norwegian Government and approval for trawling was given by the Directorate of Fisheries. No specific ethical approval was applied for 1992–2004 as this was not required at that stage. Some of the species caught are little known and the deep water redfish (Sebastes mentella) is red listed (http://www.iucnredlist.org/). The surveys have contributed to additional knowledge about these species and also provided information for area closures to protect juveniles of red fish for example.
Field Study
Data on fish species abundance and biomass for 82 fish taxa were collected during the former annual shrimp surveys conducted by the Norwegian Institute of Fisheries and Aquaculture (NIFA) and the Institute of Marine Research (IMR) in the Barents Sea from 1992 until 2004, when the shrimp survey was terminated. Survey year, vessel used, institution in charge, departure date, number of survey days and number of stations sampled are presented in Table 1. The study area ranged from 70°N to 77°N and from 15°E to 36°E, and the depth at stations sampled varied between 100 m and 495 m (Fig. 1a). Stations were placed on a grid with 20−30 nautical miles distance between stations [37].
Table 1. Survey year, vessel used, institution in charge (NIFA: Norwegian Institute of Fisheries and Aquaculture, IMR: Institute of Marine Research), departure date, number of survey days and number of stations sampled.
| Year | Institute | Vessel | Dep. Date | Nr. days | Nr.stations |
| 1992 | NIFA | M/T Gargia | 02. May | 29 | 176 |
| 1993 | NIFA | R/V Jan Mayen | 22. April | 20 | 141 |
| 1994 | NIFA | R/V Jan Mayen | 25. April | 22 | 112 |
| 1995 | NIFA | R/V Jan Mayen | 18. April | 20 | 125 |
| 1996 | NIFA | R/V Jan Mayen | 15. April | 20 | 141 |
| 1997 | NIFA | R/V Jan Mayen | 19. April | 22 | 91 |
| 1998 | NIFA | R/V Jan Mayen | 19. April | 18 | 110 |
| 1999 | NIFA | R/V Jan Mayen | 15. April | 15 | 97 |
| 2000 | NIFA | R/V Jan Mayen | 18. April | 19 | 123 |
| 2001 | NIFA | R/V Jan Mayen | 21. April | 15 | 90 |
| 2002 | IMR | R/V Jan Mayen | 16. April | 17 | 107 |
| 2003 | IMR | R/V Jan Mayen | 14. April | 21 | 109 |
| 2004 | IMR | R/V Jan Mayen | 12. April | 21 | 127 |
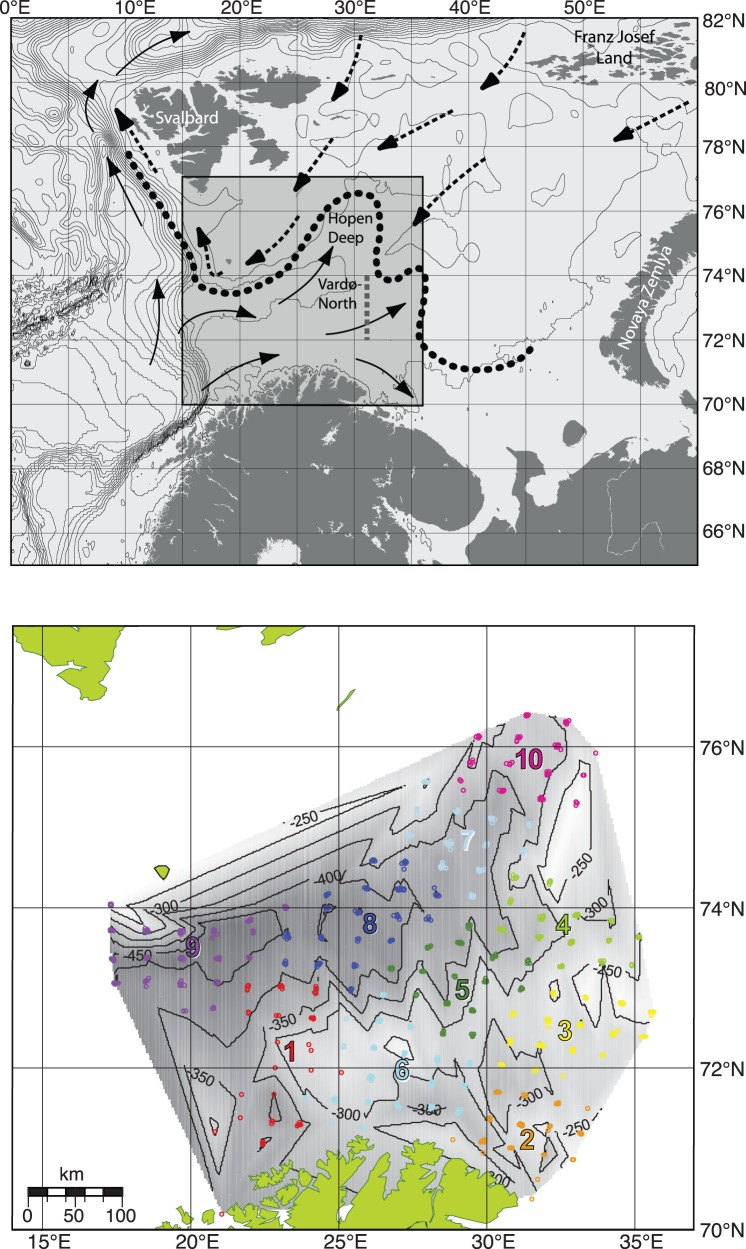
Figure 1. Main surface currents, bathymetry and sampling regions in the Barents Sea.
(a) Atlantic currents (–>) and Arctic currents (–>) and the mean position of where these water masses meet, at the depth of 20–100 m, the Polar Front (• • •) that most years follows the 200 m depth isoline that limits the study area in the North. The Vardø-North section located at 31°13′E. The study area is indicated by the grey square. In (b) bathymetry (shaded with isolines) based on information from all stations in 1992−2004 and stations (dots) with color of region (R1–R10). Stations shallower than 200 m have been excluded in all years.
A total of 1549 stations were sampled in the 13 years of shrimp surveys conducted. The station spacing and number of stations changed between years (Table 1), so that the area covered in all years was roughly the same. In order to take into account the spatial nature of the samples in the multivariate analysis, the sampling area was partitioned into 10 regions based on a k-means clustering algorithm of the distances between stations (Fig. 1b). The survey trawl (Campelen 1800), a shrimp trawl by design, is widely used in ground fish surveys (e.g. in the Barents Sea, the North Sea and off Newfoundland), and has a good catchability for demersal fish [38]. However, several species with more pelagic characteristics are also regularly caught in this trawl. The mean depth was recorded for each haul. A temperature sensor (Scanmar) was attached to the head-rope of the survey trawl to ensure a bottom temperature estimate at each station. The temperature sensor was calibrated against CTD measurements.
During the annual shrimp survey all fish were identified, counted, and total weight per species recorded. The dataset was standardized to ensure that sampling effort did not bias results between years. The 1992 station grid was used as reference. Stations closest to reference were chosen for each year, then replicate samples at stations in a year as well as stations shallower than 200 m were excluded (189 in total out of the 1549 leaving 1360 stations). There were imbalances between the 10 regions in terms of number of samples taken from year to year, and this could lead to finding inter-annual differences that are due to these sampling imbalances rather than real changes. To avoid this sampling bias, the data (abundance by species, mean weight, biomass and bottom temperature) from each station were reweighted to reflect equal representation in each region across the years. For example, in 1992 region 1 in the south-west had 17 samples whereas for all other years there were no more than 7, in some cases only one sample – the abundances in region 1 were thus down-weighted in 1992, and the under sampled regions similarly up-weighted to reflect the same level of sampling in all years.
Data Analysis
All statistical analyses were run with the software R 2.10.0 [39], using the R package vegan [40] for multivariate analyses. After the reweighting of the stations, described above, to correct for sampling disparity between years, the biomass, abundance, species number, mean weight over all species and size classes, and the Shannon–Weaver diversity index (H′) in each haul was calculated as:
| (1) |
where pi is the proportion of total abundance of species i and S is the number of species [41].
The P/D ratio was calculated from the data set as the sum of pelagic fish abundance divided by the sum of demersal fish abundance [24], [42] as a coarse metric of fish community structure. Information for the classification of species habitat was obtained from FishBase [43] and was used to define the demersal group and extend the pelagic group to include largely planktivorous species that are demersal in habit, but during night leave the bottom to feed on plankton. Species characterized as bathydemersal (habitat category “P-D” in Table 2) were excluded, and when generic names did not allow for separation between species with different habitat preference (e.g. Sebastes spp.), the species group was excluded from the P/D ratio calculation (Table 2).
Table 2. Taxa identified in the SW Barents Sea in spring 1992−2004.
| Scientific name | Abbreviation | Common name | Habitat | % ofind. | % ofstations | Yearspresent | ||||||
| PANDALIDAE | ||||||||||||
| Pandalus borealis (Krøyer, 1838) | Shrimp | 94.3 | 13 | |||||||||
| CHIMAREIDAE | ||||||||||||
| Chimaera monstrosa Linnaeus, 1758 | Rabbit fish | + | + | 1 | ||||||||
| DQUALIDAE | ||||||||||||
| Somniosus microcephalus (Bloch & Shneider, 1801) | Greenland shark | + | + | 1 | ||||||||
| RAJIDAE | ||||||||||||
| Amblyraja hyperborea (Collett, 1879) | Ra spp | Arctic skate | D | + | 0.9 | 7 | ||||||
| Amblyraja radiata (Donovan, 1808) | Ra spp | Thorny skate | D | 0.6 | 64.6 | 13 | ||||||
| Bathyraja spinicauda (Jensen, 1914) | Ra spp | Spinetail ray | D | + | 1.5 | 9 | ||||||
| Dipturus batis (Linnaeus, 1758) | Ra spp | Blue skate | D | + | + | 7 | ||||||
| Raja clavata Linnaeus, 1758 | Ra spp | Thornback ray | D | + | 2.1 | 10 | ||||||
| Rajella fyllae (Lütken, 1887) | Ra spp | Round ray | D | + | 1.7 | 10 | ||||||
| Rajidae spp | Ra spp | Skates | D | + | 3.9 | 4 | ||||||
| CLUPEIDAE | ||||||||||||
| Clupea harengus Linnaeus, 1758 | Cl ha | Herring | P | 0.8 | 24.3 | 13 | ||||||
| OSMERIDAE | ||||||||||||
| Mallotus villosus (Müller, 1776) | Ma vi | Capelin | P | 9.0 | 68.8 | 13 | ||||||
| ARGENTINIDAE | ||||||||||||
| Argentina silus (Ascanius, 1775) | Ar si | Greater argentine | P | + | 2.6 | 12 | ||||||
| Argentina sphyraena Linnaeus, 1758 | Lesser argentine | + | 0.5 | 4 | ||||||||
| STENOPYCHIDAE | ||||||||||||
| Maurolicus muelleri (Gmelin, 1789) | Ma mu | Pearlsides | + | 0.3 | 4 | |||||||
| MYCTOPHIDAE | ||||||||||||
| Benthosema glaciale (Reinhardt, 1837) | Be gl | Glacier lanternfish | P | + | 3.6 | 11 | ||||||
| PARALEPIDAE | ||||||||||||
| Arctozenus risso (Bonaparte, 1840) | Ar ri | Ribbon barracudina | P | 0.2 | 16.9 | 12 | ||||||
| GADIDAE: GADINAE | ||||||||||||
| Boreogadus saida (Lepetchin, 1774) | Bo sa | Polar cod | P | 4.1 | 26.0 | 13 | ||||||
| Gadiculus argenteus thori Schmidt, 1914 | Ga at | Silvery pout | D | + | + | 7 | ||||||
| Gadus morhua Linnaeus, 1758 | Ga mo | Cod | D | 17.1 | 95.3 | 13 | ||||||
| Melanogrammus aeglefinus (Linnaeus, 1766) | Me ae | Haddock | D | 9.5 | 71.9 | 13 | ||||||
| Merlangius merlangus (Linnaeus, 1758) | Whiting | + | + | 2 | ||||||||
| Micromesistius poutassou (Risso, 1827) | Mi po | Blue whiting | P | 2.0 | 26.4 | 13 | ||||||
| Pollachius virens (Linnaeus, 1758) | Po vi | Saithe | D | + | 6.4 | 12 | ||||||
| Trisopterus esmarkii (Nilsson, 1855) | Tr es | Norway pout | P-D | 1.9 | 24.4 | 13 | ||||||
| GADIDAE: LOTINAE | ||||||||||||
| Brosme brosme (Ascanius, 1775) | Br br | Tusk | D | + | 1.2 | 10 | ||||||
| Ciliata mustela (Linnaeus, 1758) | Five-bearded rockling | + | 0.1 | 3 | ||||||||
| Enchelyopus cimbrius (Linnaeus, 1766) | Ga spp | Four-bearded rockling | D | + | 2.4 | 10 | ||||||
| Gaidropsarus argentatus (Reinhardt, 1937) | Ga spp | Arctic rockling | + | 0.2 | 1 | |||||||
| Gaidropsarus vulgaris (Cloquet, 1824) | Ga spp | Three-bearded rockling | D | + | 0.9 | 4 | ||||||
| Molva dipterygia (Pennant, 1784) | Mo di | Blue ling | + | 0.1 | 2 | |||||||
| Molva molva (Linnaeus, 1758) | Mo mo | Ling | + | + | 1 | |||||||
| Raniceps raninus (Linnaeus, 1758) | Ra ra | Tadpole fish | + | + | 1 | |||||||
| MACROURIDAE | ||||||||||||
| Macrourus berglax Lacepéde, 1801 | Ma be | Onion-eye grenadier | D | + | 4.3 | 12 | ||||||
| ZOARCIDAE | ||||||||||||
| Gymnelus retrodorsalis Le Danois, 1913 | Ly spp | Aurora unernak | D | + | 0.4 | 3 | ||||||
| Lycenchelys kolthoffi Jensen, 1904 | Ly spp | Checkered wolf eel | D | + | 0.5 | 5 | ||||||
| Lycenchelys sarsii (Collet, 1871) | Ly spp | Sars’ wolf eel | D | + | + | 1 | ||||||
| Lycodes esmarkii Collet, 1875 | Ly es | Greater eelpout | D | 0.2 | 13.7 | 12 | ||||||
| Lycodes eudipleurostictus Jensen, 1902 | Ly eu | Doubleline eelpout | D | + | 7.7 | 12 | ||||||
| Lycodes frigidus Collet, 1879 | Ly spp | Glacial eelpout | D | + | 0.4 | 4 | ||||||
| Lycodes gracilis M. Sars, 1867* | Ly gr | Vahl’s eelpout | D | 0.7 | 53.4 | 13 | ||||||
| Lycodes pallidus Collet, 1879 | Ly spp | Pale eelpout | D | + | 1.0 | 4 | ||||||
| Lycodes reticulatus Reinhardt, 1835 | Ly spp | Arctic eelpout | D | + | 1.5 | 4 | ||||||
| Lycodes rossi Malmgren, 1865 | Ly spp | Threespot eelpout | D | + | 7.7 | 8 | ||||||
| Lycodes seminudus Reinhardt, 1937 | Ly spp | Longear eelpout | D | + | 1.0 | 5 | ||||||
| Lycodes spp | Ly spp | Eelpout (spp.) | D | + | 6.1 | 6 | ||||||
| Lycodes squamiventer Jensen, 1904 | Ly spp | Scalebelly pout | D | + | + | 1 | ||||||
| Lycodonus flagellicauda (Jensen, 1902) | Ly spp | Eelpout sp. 1 | D | + | + | 2 | ||||||
| SCORPAENIDAE | ||||||||||||
| Sebastes marinus (Linnaeus, 1758) | Se spp | Golden redfish | 2.0 | 25.8 | 13 | |||||||
| Sebastes mentella (Travin, 1951) | Se spp | Beaked redfish | 23.1 | 71.9 | 12 | |||||||
| Sebastes viviparus Krøyer, 1845 | Se spp | Norway redfish | + | 0.8 | 4 | |||||||
| Sebastes spp | Se spp | Redfish (spp.) | 6.9 | 20.5 | 8 | |||||||
| GASTEROSTERIDAE | ||||||||||||
| Gasterosteus aculeatus aculeatus (Linnaeus, 1758) | Ga aa | Three-spines stickleback | P | + | 0.8 | 4 | ||||||
| COTTIDAE | ||||||||||||
| Artediellus atlanticus Jordan & Evermann, 1898 | Ar at | Atlantic hookear sculpin | D | 0.9 | 41.5 | 13 | ||||||
| Myoxocephalus scorpius (Linnaeus, 1758) | My sc | Shorthorn sculpin | D | + | 1.1 | 7 | ||||||
| Triglops murrayi Günther, 1888 | Tr spp | Moustache sculpin | D | 0.2 | 8.1 | 13 | ||||||
| Triglops pingelii Reinhardt, 1837 | Tr spp | Ribbed sculpin | D | + | 0.9 | 2 | ||||||
| Triglops spp | Tr spp | Triglops sculpins | D | 0.1 | 4.5 | 9 | ||||||
| COTTINCULIDAE | ||||||||||||
| Cottunculus microps Collet, 1875 | Co mi | Polar sculpin | D | + | 3.9 | 12 | ||||||
| AGONIDAE | ||||||||||||
| Agonus cataphractus (Linnaeus, 1758) | Ag ca | Hook nose | + | + | 2 | |||||||
| Leptagonus decagonus (Bloch & Schneider, 1801) | Le de | Atlantic poacher | D | 0.5 | 29.3 | 13 | ||||||
| Ulcina olrikii (Lytken, 1877) | Arctic alligatorfish | + | + | 1 | ||||||||
| CYCLOPTERIDAE | ||||||||||||
| Cyclopterus lumpus Linnaeus, 1758 | Cy lu | Lumpsucker | P | + | 13.1 | 13 | ||||||
| Eumicrotremus spinosus (Fabricius, 1776) | Atlantic spiny lumpsucker | + | 0.3 | 4 | ||||||||
| LIPARIDAE | ||||||||||||
| Careproctus sp** | Ca sp | Sea snail sp. 1 | P-D | 0.1 | 26.2 | 13 | ||||||
| Liparis bathybii (Collet, 1879) | Li ba | Black seasnail | + | 0.3 | 4 | |||||||
| Liparis fabricii Krøyer 1847 | Li fa | Gelatinous seasnail | P-D | + | 3.3 | 10 | ||||||
| Liparis gibbus Bean, 1881*** | Li gi | Variegated snailfish | D | + | 1.7 | 3 | ||||||
| Liparidae spp | Li spp | Snailfishes (spp.) | + | 0.4 | 3 | |||||||
| STICHAEIDAE | ||||||||||||
| Anisarchus medius (Reinhardt, 1837) | An me | Stout eelblenny | + | 0.4 | 3 | |||||||
| Leptoclinus maculatus (Fries, 1837) | Le ma | Spotted snake blenny | D | 0.3 | 13.3 | 13 | ||||||
| Lumpenus lampraetaeformis (Walbaum,1972) | Lu la | Snake blenny | D | + | 9.5 | 12 | ||||||
| Stichaeidae spp | St spp | Pricklebacks | + | 0.3 | 2 | |||||||
| ANARHICHADIDAE | ||||||||||||
| Anarhichas denticulatus Krøyer, 1845 | An de | Northern wolffish | D | 0.2 | 39.9 | 13 | ||||||
| Anarhichas lupus Linnaeus, 1758 | An lu | Atlantic wolffish | D | + | 3.7 | 11 | ||||||
| Anarhichas minor Olafsen, 1772 | An mi | Spotted wolffish | D | + | 18.2 | 13 | ||||||
| PLEURONECTIDAE | ||||||||||||
| Glyptocephalus cynoglossus (Linnaeus, 1758) | Gl cy | Witch flounder | + | 0.9 | 8 | |||||||
| Hippoglossoides platessoides (Fabricius, 1780) | Hi pl | Long rough dab | D | 17.9 | 98.5 | 13 | ||||||
| Hippoglossus hippoglossus (Linnaeus, 1758) | Hi hi | Halibut | + | 0.1 | 1 | |||||||
| Limanda limanda (Linnaeus, 1758) | Dab | + | + | 1 | ||||||||
| Microstomus kitt (Walbaum, 1792) | Mi ki | Lemon sole | + | 0.1 | 1 | |||||||
| Pleuronectes platessa Linnaeus, 1758 | Pl pl | European plaice | + | 0.4 | 4 | |||||||
| Reinhardtius hippoglossoides (Walbaum, 1792) | Re hi | Greenland halibut | P-D | 0.8 | 60.8 | 13 | ||||||
Abbreviations are given for all taxa included in the CCA, and indicator species for the three assemblages in bold. Habitat is indicated as pelagic (P), demersal (D) or pelagic-demersal (P-D).
Percentages <0.1% denoted by +.
Lycodes gracilis M. Sars, 1867* eq Lycodes vahlii Reinhardt, 1831.
Careproctus** sp eq Careproctus derjugini Chernova 2005 and eq Careproctus reinhardthi (Krøyer,1862).
Liparis gibbus Bean, 1881*** eq Liparis liparis (Linnaeus, 1766).
The structural variation of the fish community abundance in space and time was modeled as a function of region, depth, bottom temperature and year by direct ordination, using canonical correspondence analysis (CCA). Due to some inconsistencies in identification it was appropriate to group species for the ordination analysis; the redfish Sebastes mentella, S. marinus, S. viviparous and S. spp. were all treated as one variable (Se_spp). All Rajidae (Ra_spp), all Triglops (Tr_spp) and some of the Lycodes (Ly_spp) were treated as one taxon respectively (Table 2). In addition, taxa occurring only once in the whole data set where excluded.
To unify the interpretation of the results, all environmental variables were coded as categorical variables. The discrete variable year was categorized as a set of 13 “crisp” dummy (zero/one) variables, one for each year, while continuous variables such as depth and bottom temperature were each coded into four “fuzzy” dummy variables adding up to 1, using so-called triangular membership functions [44]. This coding scheme loses no information in the data and has the advantage that nonlinear relationships can be diagnosed. The four so-called “hinge points” used in the creation of the fuzzy categories were: for depth, 206 (minimum depth), 300, 400 and 495 meters (maximum depth), and for bottom temperature.
–1.4 (minimum temperature), 1, 3 and 6.4 degrees Celsius (maximum temperature). Regional group was also coded into dummy variables in a fuzzy way so that the coding of stations on the borderline between two regions would be coded 0.5 and 0.5, for example, for the respective regions, whereas a station near the center of a region would be coded totally (1) into that region – like the coding of depth and temperature, this strategy conserves the maximum information about the geographical locations of the stations. The CCA model was tested by Monte Carlo permutation [40], [45].
Previously identified fish assemblages in the Barents Sea [30] are the cold water NE assemblage, with four key species (Artediellus atlanticus, Leptagonus decagonus, Leptoclinus maculates and Lumpenus lampraetaeformis) and the warm water S assemblage with three key species (Argentina silus, Pollachius virens and Trisopterus esmarkii). We have identified the stations where at least a total of 10 individuals of the key species of the respective assemblage were observed. Maps with S assemblage and NE assemblage stations were drawn for each year. The annual distributions of the warm water S assemblage and the cold water NE assemblage, in the period 1992 to 2004, were then integrated in one map.
Results
Temporal Change in Biomass, Abundance, Diversity and P/D-ratio
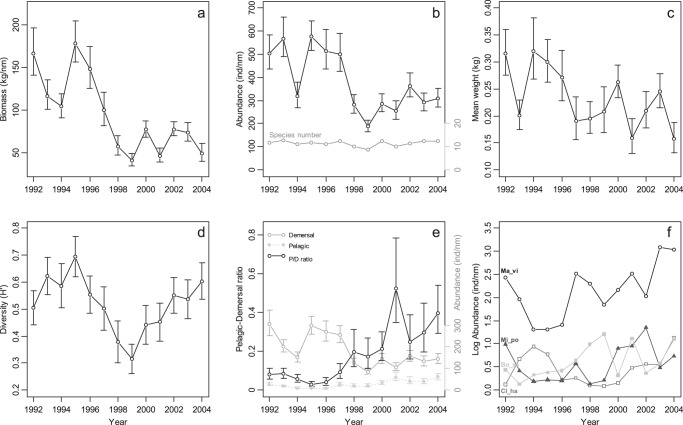
The biomass, abundance and the annual mean weight of individual fish decreased from 1996 to 1999, then increased, but remained at a lower level than prior to 1996 (Fig. 2a–c). The Shannon-Weaver diversity (H′) also declined from 1996 to 1999 but then increased again (Fig. 2d). The overall mean species number is 11.5, yet in 1999 it dropped to 8.7 (Fig. 2b), and contributes to the decline in diversity in the same year (Fig. 2d).
Figure 2. Fish community parameters for each year 1992−2004.
The mean biomass (a), mean abundance and (with scale on right) species number (b), mean weight of individual fish (c), Shannon–Weaver diversity (H’) (d), the mean P/D ratio and (with scale in the right) pelagic and demersal fish abundances (e), and mean log-transformed abundance of four pelagic species (f) Mallotus villosus (circles), Boreogadus saida (solid gray squares), Micromesistius poutassou (triangles) and Clupea harengus (open squares). The 95% confidence intervals for the means are shown in most cases based on the log-transformed data after reweighting to be representative of the sampling regions.
The P/D ratio increased (Fig. 2e), revealing that the fish community has become more dominated by small pelagics after 1996. The abundance of demersal fish including dominant species belonging to e.g. Cottidae, Rajidae, and Gadidae, (Gadus morhua and Melanogrammus aeglefinus) decreased (Fig. 2e), while that of pelagic species such as Mallotus villosus, Boreogadus saida, Micromesistius poutassou and Clupea harengus increased (Fig. 2e, 2f).
Spatial Patterns and Temporal Change in Community Structure
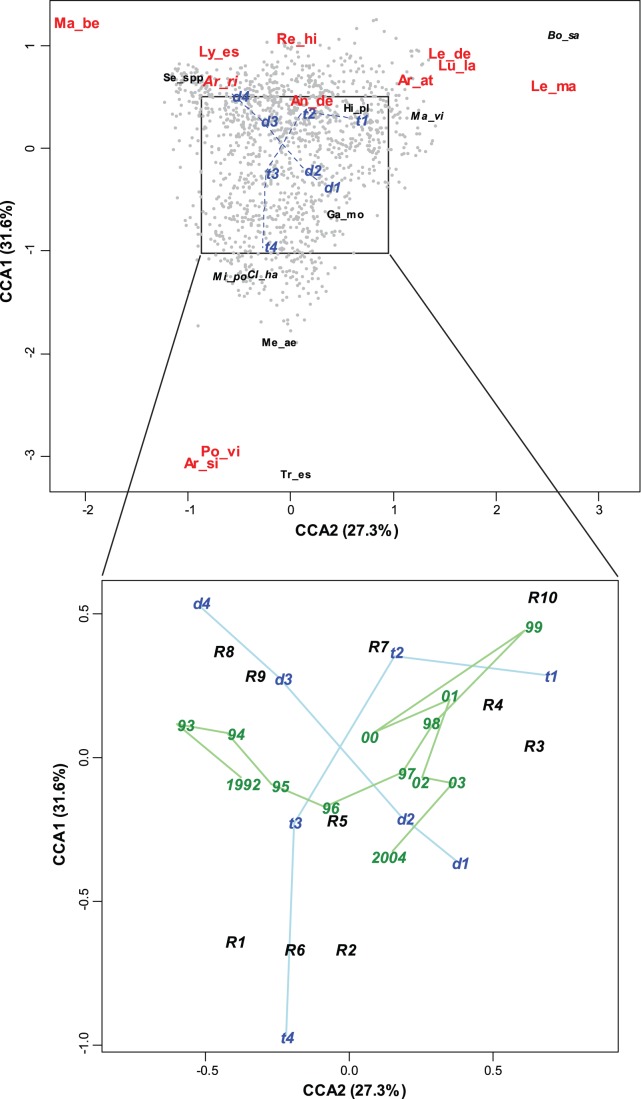
The CCA allows stations and species to be aligned with physical and temporal variables. The full set of environmental variables explains 28.3% of the variation in the species abundances, which is highly significant at P<0.0001 according to a permutation test [40]. The vertical first axis (CCA1) explains 31.6% of this constrained variation, and the horizontal second axis (CCA2) explains 27.3%.
Three distinct fish assemblages, characterized by their indicator species (key species occurring in the 13 years studied (see Table 2)) and previously defined by Fossheim et al. [30], were identified in the CCA model (Fig. 3a) as follows:
Figure 3. Canonical correspondence analysis (CCA) ordination biplot of axes 2 (horizontal) and axes 1 (vertical) of 55 fish taxa and 1360 stations in the period 1992−2004.
(a) The high-contributing species are labeled in black (small font), indicator species of the three distinct fish assemblages are labeled in red (large font), cold water NE assemblage (Ar_at = Artediellus atlanticus, Le_de = Leptagonus decagonus, Le_ma = Leptoclinus maculates and Lu_la = Lumpenus lampraetaeformis), warm water S assemblage (Ar_si = Argentina silus, Po_vi = Pollachius virens and Tr_es = Trisopterus esmarkii) and deep assemblage (Ly_es = Lycodes esmarkii, An_de = Anarhichas denticulatus and Re_hi = Reinhardtius hippoglossoides) previously defined by Fossheim et al. [30]. Pelagic species (in italics) are associated with the northern (Bo_sa = Boreogadus saida and Ma_vi = Mallotus villosus), southern (Mi_po = Micromesistius poutassou and Cl_ha = Clupea harengus) and deep assemblages (Ar_ri = Arctozenus risso). (b) Central section of Fig. 3a, showing the categories of bottom temperature (t) and depth (d), as in Fig. 3a, as well as those for the 10 regions (R) and the 13 years.
a warm water S assemblage (Argentina silus, Pollachius virens and Trisopterus esmarkii, CCA1< −3, CCA2<0),
a cold water NE assemblage (Artediellus atlanticus., Leptagonus decagonus, Leptoclinus maculates and Lumpenus lampraetaeformis, CCA1>0.5, CCA2>1) and
a deep assemblage (Arctozenus risso, Lycodes esmarkii, Anarhichas denticulatus and Reinhardtius hippoglossoides, CCA1>0.5, CCA2<0.5).
The species composition in the three assemblages did not change over time. Pelagic species such as Boreogadus saida and Mallotus villosus were associated with the cold water NE assemblage and Micromesistius poutassou and Clupea harengus were associated with the warm water S assemblage.
All variables in the CCA map are depicted by points representing categories; either years, regions (R), or ordinal categories of depth and bottom temperature (Fig. 3a, b). In the case of all variables except year, the coding is fuzzy, but the interpretation of all categories is the same. Each one of the station samples, depicted as grey dots in Fig. 3a, has a category of each variable associated with it. So each category can be displayed at the average of the station points having that particular category, using weighted averaging for the fuzzy categories and ordinary averaging for the years. Thus year 1992 appears at the lower left of the graphic because it is the average point of all the 1992 stations which must have been generally situated towards the lower left hand side (Fig. 3b). Similarly, the lowest bottom temperature category (t1) is situated at top right of the display, because it is the average of all the stations that have the lowest set of temperatures. Region 10, in the north-east of the sampling region (Fig. 1b), falls in the upper right quadrant of the display because it is the average position of all sampling points in that region (Fig. 3b). Depth turns out to be a set of points in a straight line, whereas temperature has a curved trajectory, showing that shallower samples occur in both low and high temperature regions, a fact that can be deduced from the map in Fig. 1b, which shows shallow depths in northern and southern regions. A strictly linear coding, which is the usual approach in CCA, would not be able to reflect this fact. The benefits of fuzzy coding are that nonlinear relationships with the environmental variables can be revealed, more variance can be explained in the species abundances, and the interpretation of the ordination triplots is unified because all variables are coded as categories [46].
The CCA map clearly separated the years until 1996 (CCA2<0) and the colder years after 1996 (CCA2>0) (Fig. 3). This indicates some change in the community structure, represented by CCA2 that shows change towards a community with more capelin and Polar cod from 1996 to 1999. Although years 2000 and 2004 get close to an “average” species composition the community does not seem to recover to the state of the early 90s (Fig. 3b). There was a significant difference in abundance, biomass, mean fish weight, P/D ratio and CCA2 values between the periods 1992−1997 and 1998−2004 (Student’s t-test P<0.01).
Discussion
Spatial Variation in the Fish Community
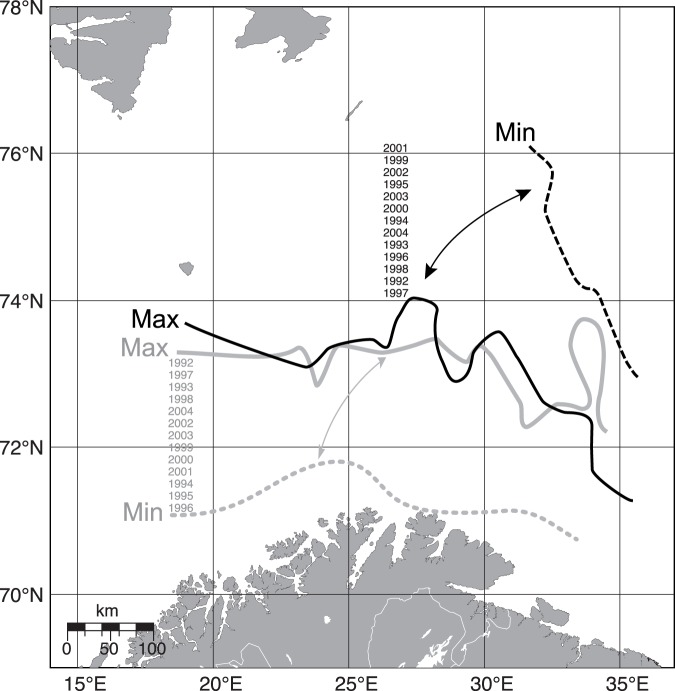
Hydrographical features such as water masses, fronts and residual currents as well as bathymetry seem to shape rather stable bottom fish assemblages in the North Atlantic [36], [47]. However, these fish assemblages may change their range of distribution along the latitude and depth gradients with an increase in temperature [48], [49]. The three fish assemblages identified in this study, a deep water assemblage, a warm water S assemblage, both associated with Atlantic water, and a cold water NE assemblage associated with mixed water (<2°C) [50], were identified in the period 1992−2004. These three assemblages have the same key species as previously identified for fish assemblages in the Barents Sea [30], and follow the same zoogeographical groupings identified in previous studies [28], [30], [51]. Hence, the species assemblages are identified over longer time periods than the 13 years studied here. Although the species may change their distribution within the 13 years studied, the S and the NE assemblages seem rather stable although they oscillate back and forth and may meet across the Barents Sea along the 2°C temperature isoline often occurring at 73–74°N (Fig. 4). Yet, the two assemblages show no consistent northwards movement as seen in neighboring North Sea fish species [25], [36]. The water mass distribution and characteristics in the Barents Sea have a major influence on the production processes [50]. Thereby the Polar Front, where Atlantic and Arctic water masses meet, and which is associated with the 200 m depth isoline in the western Barents Sea, constitutes a clear zoogeographical boundary [52]. An Arctic fish assemblage described recently by Johannesen et al. [34] and located north of the Polar Front and thereby north of our study area has many species in common with the NE assemblage.
Figure 4. Distribution of assemblages.
Maximum and minimum distribution of the cold water NE assemblage (Artediellus atlanticus, Leptagonus decagonus, Leptoclinus maculates and Lumpenus lampraetaeformis) in black and the warm water S assemblage (Argentina silus, Pollachius virens and Trisopterus esmarkii) in grey. Max indicates the widest distribution of respective assemblage (area where ≥10 individuals of the key species group have been observed over time), and Min indicates the narrowest annual distribution of both key species groups in 1992 to 2004. The ranks of years indicate the position of the maximum and the minimum distribution of the S and NE assemblages each year.
Temporal Change
In the Barents Sea the winter 1996−1997 was the coldest since 1989 [53] (Fig. 5a), and resulted in a dramatic reduction in primary production in 1998 [54], high mortality of recruits, e.g. shrimp [55], and reduction in zooplankton biomass in the same period. At the same time the herring (Clupea harengus) stock, that migrates into the Barents Sea for food, doubled in size [56]. The feeding by herring in the pelagic layer may have resulted in less food available for demersal species and thereby a biomass reduction in the fish community (Fig. 2a). There is a potential competition between herring and cod juveniles as they have a dietary as well as a temporal and spatial overlap [57], [58], and the presence of juvenile herring reduces capelin recruitment and hence food availability in the Barents Sea [59]. In years with many capelin, these are preyed upon by cod, herring, haddock (Melanogrammus aeglefinus) and other demersal fish [60], such as Greenland halibut (Reinhardtius hippoglossoides) [61] and skates [62].
Figure 5. Temperature, NAO index and demersal fish landings.
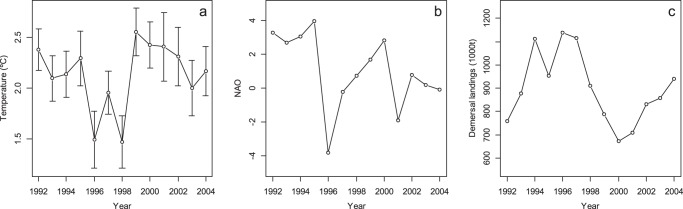
Mean bottom temperature (measured with Scanmar sensor attached to the survey trawl) with 95% confidence interval (a), the NAO index from the National Centre of Atmospheric Research, USA [80] (b) and demersal fish landings (Gadus morhua, Melanogrammus aeglefinus, Pollachius virens, Reinhardtius hippoglossoides, Sebastes marinus and Sebastes mentella) in the Barents Sea (ICES subareas I and II) [81] (c).
Also the high annual demersal fish landings in the early 90s had a negative effect on the fish abundance and the demersal fish abundance declined to 1/3 from 1995 to 1999 (Fig. 2e, 5d).
As in North Icelandic waters the fish biodiversity decreased after 1996 [36] (Fig. 2d). The species abundance and biomass decreased from 1996 to 1999 (Fig. 2a, 2b) and the mean fish weight declined (Fig. 2c, 2e). Thereby the community structure changed and the P/D-ratio increased as demersal fish abundance declined and pelagic species became more abundant (Fig. 2e, 2f). The low bottom temperature influenced species distribution, and opportunistic seasonal migrants, e.g., Mallotus villosus and Boreogadus saida or Clupea harengus and Micromesistius poutassou increased in cold and warm conditions respectively.
Species that change their distribution rapidly are known to have faster lifecycles and smaller body sizes than non-shifting species [25], which may explain some of the change to more pelagic species in the Barents Sea. This kind of temperature-driven change has been observed in the Northwest Atlantic [63], in the North Sea [25], in Narragansett Bay [24] and off California [64]. These studies point to climate variability as the main reason for fish community change.
The Barents Sea is a low-diversity Arctic system compared to the species-rich, temperate North Sea [65], [66]. The low species richness and diversity is a result of low sea temperature, a relation that is also found in other areas of the North Atlantic [65], [67]. Low diversity ecosystems are less resilient than high diversity ecosystems, since species loss may lead to empty niches [67]. A reduction in diversity reduces the ability of the ecosystem to compensate for change [68], and Arctic ecosystems are therefore considered to have low resilience [65]. We identified a dramatic drop in diversity from 1996 to 1999, partly driven by reduced species richness, and this is also an indication of low resilience in the fish community. Although the diversity and the abundance increased after 1999 the average fish size remains small and the pelagic species thrive (Fig. 2a–f).
Regime Shifts in the Barents Sea?
Fish assemblages in the Barents Sea seem to have the same characteristic species over time and are determined by depth and temperature [69]–[72], but structural changes may still happen. These kinds of changes are often detected and reported when the change is obvious and a regime shift has already occurred [22]–[24]. In the Barents Sea an ecological regime shift in the 1920s was caused by warmer than normal sea temperatures, reduced sea ice coverage and enhanced Atlantic inflow [7]. Like in the 1920s, a climate change was identified in the early 1980s documented by an increase in the NAO index and temperature; however, a response in increased fish biomass as observed in the 1920s did not follow. Since the demersal fish biomass reduction in the 1960s, the large fish stocks have oscillated at a lower level [73]. This may be attributable to high fishing activity or change in oceanographic conditions, which is often observed as low primary production, or a combination of both fishing and change in climatic conditions [10].
Lees et al. [10] expected an ecological regime shift in the Barents Sea as a consequence of the climatic regime shift in 1995−1998 (decline in NAO and sea temperature), but the lack of response (decline) in the ecosystem indicators such as zooplankton biomass, gadoid recruitment and gadoid spawning stock biomass was considered to be a delay in ecological response [10]. In an ecosystem experiencing a regime shift, individual species may not respond or their response may be slow or lagged, particularly in long-lived species [6], [21], and the response by commercial species is likely to be masked by fishing mortality. We argue that change in other attributes of the ecosystem, such as fish biomass, mean individual weight, P/D ratio, and species composition, indicate significant community-wide changes in the Barents Sea, probably as a response to observed changes in bottom temperature the NAO index and fishing mortality (Fig. 5a–c). However, the time period studied here is too short to conclude that the change in fish community parameters observed in the Barents Sea between 1996 and 1999 is a more persistent (>10 year) regime shift.
Low resilience due to high fishing mortality in the mid-90s, combined with a sudden oceanographic change in 1996 seems to have resulted in an ecological change. Although the drivers, temperature, NAO index, and fishing mortality moved back to pre-change conditions, the fish community had not recovered to its previous state in 2004 (Fig. 2a–d).
Drivers
Successful management of marine ecosystems demands a good understanding of species interactions and fisheries as well as the effect of variation in oceanographic conditions [74]. The annual catch of demersal species including cod, haddock, saithe (Pollachius virens) and others increased from 360 000 tons in 1990 to over 1 million tons in 1994 and stayed at this high level until 1998 [3] (Fig. 5c). The P/D ratio seems to respond with a steady increase from 1996 to 1999, mainly due to the removal of commercial demersal fish. The P/D ratio then continues to increase as recruitment of demersal fish stays low, probably due to reduced spawning stock biomass and low temperatures, and as the pelagics increase probably due to reduced predation pressure and fast lifecycles (Fig. 2e). The demersal fisheries targeting fish at a high trophic level are likely to have reduced the resilience in the Barents Sea ecosystem as seen in the NW Atlantic [65]. We hypothesize that intense fishing first reduced the resilience in the ecosystem, hence when a change in climatic conditions reduced temperatures and production, the fish community responded with a further decline in abundance and average fish weight. Yet, as fisheries and change in oceanographic conditions affect the fish community at the same time in different ways, it is not possible to separate between effects induced by the two drivers [14].
Recovery
Although we observe a rapid decline in fish biomass, abundance and mean size and an increase in small pelagics, no obvious change in the commercial fish stocks of the Barents Sea has been observed in the study period, and the fish community may be able to recover to the composition observed in the early 1990s, characterized by relatively high abundance, high diversity and more demersal species as recruitment to large fish stocks is likely to increase in warm years [75]. However, such a re-establishment may be hindered by further increasing temperatures, as the Barents Sea is turning into a more North Sea like pelagic-dominated ecosystem [66], [76]. Forecasts predict a temperature increase that is believed to result in a primary production increase followed by a higher fish production in the Barents Sea [13], [77]–[79]. Yet, the long-term effects of warming in the Barents Sea are uncertain [50], as these studies do not evaluate the interactions between species and may therefore turn out to be too optimistic.
Conclusions
Although the Barents Sea fish assemblages identified herein have not changed their distributions markedly, structural changes have been observed, including reduced fish biomass, abundance and mean-weight as well as increased P/D-ratio. These community characteristics did not seem to recover as the drivers, oceanographic conditions and fishing, changed back to the previous stage. A recovery may be hindered by increasing temperatures and the demersal fishery, which are transforming the Barents Sea into a more North Sea like, pelagic-dominated ecosystem.
Acknowledgments
We want to thank the crew on board R/V Helmer Hanssen (earlier R/V Jan Mayen) for assisting us through years of surveys. Frøydis Strand kindly helped us with the figures. Randi Ingvaldsen assisted us with the present knowledge on oceanographic conditions in the Barents Sea. We also thank two anonymous referees and the editor who contributed to improving both the scientific content and language of the paper.
Funding Statement
The data were collected during the former annual shrimp survey conducted by the Norwegian Institute of Fisheries and Aquaculture (NIFA) and the Institute of Marine Research (IMR) in the Barents Sea from 1992 until 2004, and were funded by the Norwegian Ministry of Fisheries and Coastal Affairs. The scientific work was also funded by the Norwegian Government through the above mentioned ministry and through the Ministry of Education and Research. Michael Greenacre's research was partially funded by the BBVA Foundation in Madrid and a grant from the Spanish Ministry of Education MTM2012-37195. The funders had no role in study design, data collection and analysis, decision to publish or the preparation of the manuscript.
References
- 1.Anon. (2002) St.meld.nr. 12 (2001–2002) Rent og rikt hav (havmiljømeldingen). Ministry of Environment, Oslo (available in English). 104 p.
- 2.Anon. (2006) St.meld.nr 8 (2005–2006) Helhetlig forvaltning av det marine miljø i Barentshavet og havområdene utenfor Lofoten (forvaltningsplan) Ministry of Environment, Oslo (available in English). 141 p.
- 3.ICES (2011) Report of the Arctic Fisheries Working Group. 678 p.
- 4. Spielhagen RF, Werner K, Sørensen SA, Zamelczyk K, Kandiano E, et al. (2011) Enhanced Modern Heat Transfer to the Arctic by Warm Atlantic Water. Science 331: 450–453. [DOI] [PubMed] [Google Scholar]
- 5.ACIA (2005) Arctic Climate Impact Assessment. Cambridge: Cambridge University Press. 1042 p.
- 6. Beaugrand G (2004) The North Sea regime shift: evidence, causes, mechanisms and consequences. Progress In Oceanography 60: 245–262. [Google Scholar]
- 7. Drinkwater KF (2006) The regime shift of the 1920s and 1930s in the North Atlantic. Progress in Oceanography 68: 134–151. [Google Scholar]
- 8. Cheung WWL, Lam VWY, Sarmiento JL, Kearney K, Watson R, et al. (2010) Large-scale redistribution of maximum fisheries catch potential in the global ocean under climate change. Global Change Biology 16: 24–35. [Google Scholar]
- 9. Mueter FJ, Litzow MA (2008) Sea ice retreat alters the biogeography of the Bering Sea continental shelf. Ecological Applications 18: 309–320. [DOI] [PubMed] [Google Scholar]
- 10. Lees K, Pitois S, Scott C, Frid C, Mackinson S (2006) Characterizing regime shifts in the marine environment. Fish and Fisheries 7: 104–127. [Google Scholar]
- 11. Genner MJ, Sims DW, Wearmouth VJ, Southall EJ, Southward AJ, et al. (2004) Regional climatic warming drives long-term community changes of British marine fish. Proceedings of the Royal Society of London Series B-Biological Sciences 271: 655–661. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Anon (2010) Survey report from the joint Norwegian/Russian ecosystem survey in the Barents Sea August-September 2010. IMR/PINRO Joint Report Series 4: 108. [Google Scholar]
- 13. Jennings S, Brander K (2009) Predicting the effect of climate change on marine communities and the consequences for fisheries. Journal of Marine Systems 79: 418–426. [Google Scholar]
- 14. Planque B, Fromentin JM, Cury P, Drinkwater KF, Jennings S, et al. (2010) How does fishing alter marine populations and ecosystems sensitivity to climate? Journal of Marine Systems 79: 403–417. [Google Scholar]
- 15. Frank KT, Petrie B, Choi JS, Leggett WC (2005) Trophic cascades in a formerly cod-dominated ecosystem. Science 308: 1621–1623. [DOI] [PubMed] [Google Scholar]
- 16. Scheffer M, Carpenter S, Foley JA, Folke C, Walker B (2001) Catastrophic shifts in ecosystems. Nature 413: 591–596. [DOI] [PubMed] [Google Scholar]
- 17. Scheffer M, Carpenter SR (2003) Catastrophic regime shifts in ecosystems: linking theory to observation. Trends in Ecology & Evolution 18: 648–656. [Google Scholar]
- 18. Hsieh CH, Glaser SM, Lucas AJ, Sugihara G (2005) Distinguishing random environmental fluctuations from ecological catastrophes for the North Pacific Ocean. Nature 435: 336–340. [DOI] [PubMed] [Google Scholar]
- 19. Overland J, Rodionov S, Minobe S, Bond N (2008) North Pacific regime shifts: Definitions, issues and recent transitions. Progress In Oceanography 77: 92–102. [Google Scholar]
- 20. Daskalov GM, Grishin AN, Rodionov S, Mihneva V (2007) Trophic cascades triggered by overfishing reveal possible mechanisms of ecosystem regime shifts. Proceedings of the National Academy of Sciences of the United States of America 104: 10518–10523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. deYoung B, Barange M, Beaugrand G, Harris R, Perry RI, et al. (2008) Regime shifts in marine ecosystems: detection, prediction and management. Trends in Ecology & Evolution 23: 402–409. [DOI] [PubMed] [Google Scholar]
- 22. Choi JS, Frank KT, Leggett WC, Drinkwater K (2004) Transition to an alternate state in a continental shelf ecosystem. Canadian Journal of Fisheries and Aquatic Sciences 61: 505–510. [Google Scholar]
- 23. Shackell NL, Frank KT (2007) Compensation in exploited marine fish communities on the Scotian Shelf, Canada. Marine Ecology Progress Series 336: 235–247. [Google Scholar]
- 24. Collie JS, Wood AD, Jeffries HP (2008) Long-term shifts in the species composition of a coastal fish community. Canadian Journal of Fisheries and Aquatic Sciences 65: 1352–1365. [Google Scholar]
- 25. Perry AL, Low PJ, Ellis JR, Reynolds JD (2005) Climate change and distribution shifts in marine fishes. Science 308: 1912–1915. [DOI] [PubMed] [Google Scholar]
- 26. Hsieh CH, Kim HJ, Watson W, Di Lorenzo E, Sugihara G (2009) Climate-driven changes in abundance and distribution of larvae of oceanic fishes in the southern California region. Global Change Biology 15: 2137–2152. [Google Scholar]
- 27.Zenkewitch L, editor (1963) The Biology of the Seas of the USSR. Moscow: Academy of Science of the USSR. 739 p.
- 28.Burgos GE (1989) The bottom fish community of the Barents Sea in the winters 1984 to 1987. [Master of philosophy]. Bergen: University of Bergen. 76 p.
- 29. Nilssen EM, Hopkins CCE (1992) Regional variability in fish-prawn communities and catches in the Barents Sea, and their relationship to the environment. ICES Marine Science Symposium 195: 331–348. [Google Scholar]
- 30. Fossheim M, Nilssen EM, Aschan M (2006) Fish assemblages in the Barents Sea Marine Biology Research. 2: 260–269. [Google Scholar]
- 31. Byrkjedal I, Høines A (2007) Distribution of demersal fish in the south-western Barents Sea. Polar Research 26: 135–151. [Google Scholar]
- 32. Dolgov AV (2007) Spatial and temporal dynamics of the demersal fish community in the Barents Sea ICES CM E. 31: 17. [Google Scholar]
- 33.Bogstad B, Aglen A, Dolgov AV, Drevetnyak KV, Gjøsæter H, et al.. (2008) Fish. In: Stiansen JE, Filin AA, editors. Joint PINRO/IMR Report on the state of the Barents Sea ecosystem 2007, with expected situation and consideration for management: IMR/PINRO Joint Report Series.
- 34. Johannesen E, Høines ÅS, Dolgov AV, Fossheim M (2012) Demersal Fish Assemblages and Spatial Diversity Patterns in the Arctic-Atlantic Transition Zone in the Barents Sea. PLoS ONE 7: e34924. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Kaiser MJ, Austen MCV, Ojaveer H (2004) European biodiversity action plan for fisheries: issues for non-target species. Fisheries Research 69: 1–6. [Google Scholar]
- 36. Stefansdottir L, Solmundsson J, Marteinsdottir G, Kristinsson K, Jonasson JP (2010) Groundfish species diversity and assemblage structure in Icelandic waters during recent years of warming. Fisheries Oceanography 19: 42–62. [Google Scholar]
- 37. Harbitz A, Aschan M, Sunnana K (1998) Optimal effort allocation in stratified, large area trawl surveys, with application to shrimp surveys in the Barents Sea. Fisheries Research 37: 107–113. [Google Scholar]
- 38. Aschan M, Sunnanå K (1997) Evaluation of the Norwegian shrimp surveys conducted in the Barents Sea and the Svalbard area 1980–1997. ICES CM 1997 Y 7: 23. [Google Scholar]
- 39.R Development Core Team (2009) R: A language and environment for statistical computing, version R 2.10.0. http://www.R-project.org.: R foundation for Statistical Computing, Vienna, Austria.
- 40.Oksanen J (2011) Multivariate Analysis of Ecological Communities in R: vegan tutorial, 42 pp.
- 41.Krebs CJ (1989) Ecological Methodology. New York: HarperCollins.
- 42. Moreno JID, Agostini VN, Caddy JF, Carocci F (2000) Is the pelagic-demersal ratio from fishery landings a useful proxy for nutrient availability? A preliminary data exploration for the semi-enclosed seas around Europe. ICES Journal of Marine Science 57: 1091–1102. [Google Scholar]
- 43.Froese R, Pauly D (2007) FishBase. www.fishbase.org : World Wide Web electronic publication.
- 44. Aşan Z, Greenacre M (2011) Biplots of fuzzy coded data. Fuzzy Sets and Systems 183: 57–71. [Google Scholar]
- 45.Legendre P, Legendre L (1998) Numerical Ecology. Amsterdam: Elsevier. 853 p.
- 46.Greenacre M (2013) Fuzzy coding in constrained ordinations. 15 p. [DOI] [PubMed]
- 47. Ehrich S, Stelzenmuller V, Adlerstein S (2009) Linking spatial pattern of bottom fish assemblages with water masses in the North Sea. Fisheries Oceanography 18: 36–50. [Google Scholar]
- 48. Dulvy NK, Rogers SI, Jennings S, Stelzenmüller V, Dye SR, et al. (2008) Climate change and deepening of the North Sea fish assemblage: a biotic indicator of warming seas. Journal of Applied Ecology 45: 1029–1039. [Google Scholar]
- 49. Poulard JC, Blanchard F (2005) The impact of climate change on the fish community structure of the eastern continental shelf of the Bay of Biscay. ICES Journal of Marine Science 62: 1436–1443. [Google Scholar]
- 50.Johannesen E, Ingvaldsen RB, Bogstad B, Dalpadado P, Eriksen E, et al.. (2012) Changes in Barents Sea ecosystem state, 1970–2009: climate fluctuations, human impact, and trophic interactions. ICES Journal of Marine Science: Journal du Conseil.
- 51.Ekman S (1953) The boreal fauna of the North Atlantic. Zoogeography of the sea. London: Sidgwick and Jackson. 100–141.
- 52. Bergstad OA, Bjelland O, Gordon JDM (1999) Fish communities on the slope of the eastern Norwegian Sea. Sarsia 84: 67–78. [Google Scholar]
- 53. Gjøsæter H, Huse G, Robberstad Y, Skogen M (2008) Havets ressurser og miljø 2008. Fisken og havet, Særn 1: 200. [Google Scholar]
- 54. Ellingsen IH, Dalpadado P, Slagstad D, Loeng H (2008) Impact of climatic change on the biological production in the Barents Sea. Climatic Change 87: 155–175. [Google Scholar]
- 55. Aschan M, Ingvaldsen R (2009) Recruitment of shrimp (Pandalus borealis) in the Barents Sea related to spawning stock and environment. Deep Sea Research Part II: Topical Studies in Oceanography 56: 2012–2022. [Google Scholar]
- 56. Skogen M, Gjøsæter H, Thoresen R, Robberstad YE (2007) The resources and environment of the sea. Fisken og havet, Særn 1: 191. [Google Scholar]
- 57. Pedersen T, Fossheim M (2008) Diet of 0-group stages of capelin (Mallotus villosus), herring (Clupea harengus) and cod (Gadus morhua) during spring and summer in the Barents Sea. Marine Biology 153: 1037–1046. [Google Scholar]
- 58. Olsen E, Aanes S, Mehl S, Holst JC, Aglen A, et al. (2010) Cod, haddock, saithe, herring, and capelin in the Barents Sea and adjacent waters: a review of the biological value of the area. ICES Journal of Marine Science: Journal du Conseil 67: 87–101. [Google Scholar]
- 59. Hallfredsson EH, Pedersen T (2009) Effects of predation from juvenile herring (Clupea harengus) on mortality rates of capelin (Mallotus villosus) larvae. Canadian Journal of Fisheries and Aquatic Sciences 66: 1693–1706. [Google Scholar]
- 60.Dommasnes A, Christensen W, Ellertsen B, Kvamme C, Melle W, et al.. (2002) An ECOPATH model for the Norwegian and Barents Sea. In: Guenette S, Christensen V, Pauly D, editors. Fisheries Impact on North Atlantic ecosystems: Models and Analyses. University of British Colombia, Vancouver: Fisheries Centre Research Reports. 213–240.
- 61. Hovde SC, Albert OT, Nilssen EM (2002) Spatial, seasonal and ontogenetic variation in diet of Northeast Arctic Greenland halibut (Reinhardtius hippoglossoides). ICES Journal of Marine Science: Journal du Conseil 59: 421–437. [Google Scholar]
- 62. Dolgov AV (2005) Feeding and food consumption by the Barents Sea skates. Journal of Northwest Atlantic Fishery Science 35: 495–503. [Google Scholar]
- 63. Murawski SA (1993) Climate change and marine fish distributions: Forecasting from historical analogy. Transactions of the American Fisheries Society 122: 647–658. [Google Scholar]
- 64. Hsieh CH, Reiss CS, Hewitt RP, Sugihara G (2008) Spatial analysis shows that fishing enhances the climatic sensitivity of marine fishes. Canadian Journal of Fisheries and Aquatic Sciences 65: 947–961. [Google Scholar]
- 65. Frank KT, Petrie B, Shackell NL (2007) The ups and downs of trophic control in continental shelf ecosystems. Trends in Ecology & Evolution 22: 236–242. [DOI] [PubMed] [Google Scholar]
- 66. Loeng H, Drinkwater K (2007) An overview of the ecosystems of the Barents and Norwegian Seas and their response to climate variability. Deep-Sea Research Part II-Topical Studies in Oceanography 54: 2478–2500. [Google Scholar]
- 67. Levin SA, Lubchenco J (2008) Resilience, robustness, and marine ecosystem-based management. Bioscience 58: 27–32. [Google Scholar]
- 68. Folke C, Carpenter S, Walker B, Scheffer M, Elmqvist T, et al. (2004) Regime shifts, resilience, and biodiversity in ecosystem management. Annual Review of Ecology Evolution and Systematics 35: 557–581. [Google Scholar]
- 69. Duplisea DE, Blanchard F (2005) Relating species and community dynamics in an heavily exploited marine fish community. Ecosystems 8: 899–910. [Google Scholar]
- 70. Gaertner JC, Bertrand JA, de Sola LG, Durbec JP, Ferrandis E, et al. (2005) Large spatial scale variation of demersal fish assemblage structure on the continental shelf of the NW Mediterranean Sea. Marine Ecology Progress Series 297: 245–257. [Google Scholar]
- 71. Gaertner JC, Bertrand JA, Souplet A (2002) STATIS-CoA: A methodological solution to assess the spatio-temporal organization of species assemblages. Application to the demersal assemblages of the French Mediterranean Sea. Scientia Marina 66: 221–232. [Google Scholar]
- 72. Mahon R, Brown SK, Zwanenburg KCT, Atkinson DB, Buja KR, et al. (1998) Assemblages and biogeography of demersal fishes of the east coast of North America. Canadian Journal of Fisheries and Aquatic Sciences 55: 1704–1738. [Google Scholar]
- 73. Gjøsæter H, Bogstad B, Tjelmeland S (2009) Ecosystem effects of the three capelin stock collapses in the Barents Sea. Marine Biology Research 5: 40–53. [Google Scholar]
- 74. Hjermann DØ, Bogstad B, Eikeset AM, Ottersen G, Gjøsæter H, et al. (2007) Food web dynamics affect Northeast Arctic cod recruitment. Proceedings of the Royal Society B-Biological Sciences 274: 661–669. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75. Stige LC, Ottersen G, Dalpadado P, Chan KS, Hjermann DO, et al. (2010) Direct and indirect climate forcing in a multi-species marine system. Proceedings of the Royal Society B-Biological Sciences 277: 3411–3420. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Yaragina N, Dolgov A (2009) Resilienc in the Norwegian and Barents Sea ecosystems. Deep Sea Research.
- 77. Drinkwater KF, Loeng H, Megrey BA, Bailey N, Cook RM (2005) The Influence of Climate Change on North Atlantic Fish Stocks - Proceedings of an ICES Symposium held in Bergen, Norway - 11–14 May 2004. ICES Journal of Marine Science 62: 1203–1204. [Google Scholar]
- 78.Cheung WWL, Lam VWY, Pauly D (2008) Modeling present and climate-shifted distribution of marine fishes and invertebrates. Vancouver: University of British Columbia. 72 p.
- 79. Drinkwater KF (2011) The influence of climate variability and change on the ecosystems of the Barents Sea and adjacent waters: Review and synthesis of recent studies from the NESSAS Project. Progress in Oceanography 90: 47–61. [Google Scholar]
- 80. Hurrell JW (1995) Decadal trends in the North-Atlantic Oscillation - Regional Temperatures and precipitation. Science 269: 676–679. [DOI] [PubMed] [Google Scholar]
- 81. ICES (2008) Report of the Arctic Fisheries Working Group. ICES CM 2008 ACOM 01: 530. [Google Scholar]