Figure 4.
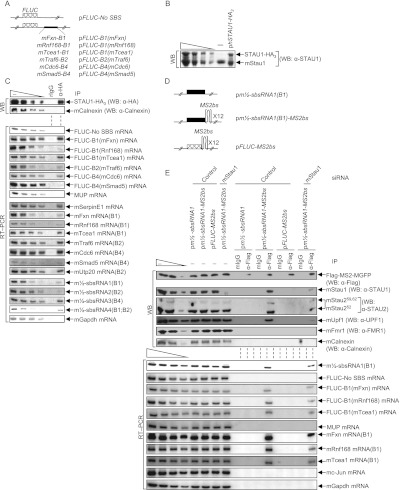
Confirmation that the predicted m½-sbsRNA–mRNA duplexes function as SBSs in C2C12 MBs. (A) Diagrams of pFLUC reporter plasmids, the 3′ UTR of which contains no SBS or the denoted mRNA 3′ UTR SINE situated 224 nt downstream from the FLUC termination codon. The cross-hatched box represents the FLUC open translational reading frame. (B) Western blotting using lysates of C2C12 MBs prior to immunoprecipitation. MBs (4 × 106 per 150-mm dish) had been transiently transfected with 10 μg of pSTAU1-HA3 or pUC19, 1 μg of each of the seven FLUC reporter plasmids, and 2 μg of phCMV-MUP and formaldehyde-cross-linked prior to lysis. (C) Western blotting (top) or RT–PCR (bottom) of lysates analyzed in B before (−) or after immunoprecipitation (IP) using anti-HA (α-HA) or, as a control for nonspecific immunoprecipitation, mIgG. (D) Diagrams of plasmids encoding m½-sbsRNA1(B1), m½-sbsRNA1(B1) harboring 12 copies of the MS2bs, or FLUC mRNA harboring 12 copies of the MS2bs. (E) Western blotting (top) or RT–PCR (bottom) before (−) or after immunoprecipitation of lysates of formaldehyde-cross-linked C2C12 MBs (4 × 106 per 150-mm dish) that had been transiently transfected with 50 nM specified siRNA and, 1 d later, with 5 μg of pFlag-MS2-hMGFP, 1 μg of each of the seven reporter plasmids shown in A, 2 μg of phCMV-MUP, and the denoted plasmid encoding m½-sbsRNA1(B1), m½-sbsRNA1(B1)-MS2bs, or FLUC-MS2bs. Immunoprecipitations were performed using anti-Flag or mIgG. All results are representative of at least two independently performed experiments (Supplemental Fig. 4; Supplemental Table 4).
