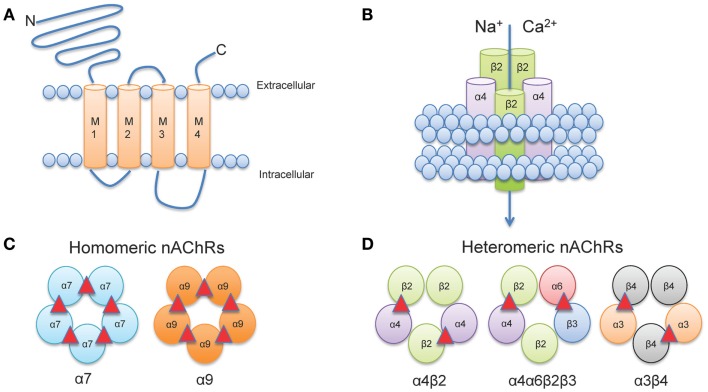
Figure 1.
Neuronal nAChR Structure. (A) Membrane topology of a neuronal nAChR subunit. Each nAChR subunit contains four transmembrane domains (M1-M4), an extracellular amino- and carboxy-terminus, and a prominent M3-M4 intracellular loop of variable length. (B) Five subunits coassemble to form a functional subunit. (C) Homomeric receptors consist of α subunits only and usually have low affinity for agonist. To date, only mammalian α7, α9, and α10 (not shown) subunits may form functional homomers. (D) The majority of high affinity nAChRs are heteromeric and consist of a combination of α and β subunits. Importantly, multiple α subunits may coassemble with multiple β subunits in the pentameric nAChR complex (illustrated here by α4α6β3β2). ACh binding sites are depicted as red triangles.