Abstract
Objective
To describe site capability and experience of the CHEER network (Creating Healthcare Excellence through Education and Research) to rapidly collect descriptive data on tinnitus and dizziness patients visiting participating CHEER sites.
Study design
Prospective observational data collection study over 6 months.
Setting
21 community otology and otolaryngology practices in the United States.
Subjects and Methods
As proof of concept, a data collection study was developed for tinnitus and dizziness patients (presenting with or without associated migraine) through a collaborative effort of the CHEER principal investigator (PI) and co-PIs. The 9-page questionnaire included validated instruments and additional patient and physician-reported information. Information was captured electronically via REDCap by each site’s CHEER research coordinator. Site initiation, data entry rates, and research coordinator feedback were also collected.
Results
Of the 21 CHEER sites, 15 participated in the study. Nine sites entered a patient within the first 31 days of study initiation and all 15 sites were entering patients and corresponding clinical data within 72 days. During the 6-month study, 1044 patients were entered into the REDCap database. Research coordinator engagement was a major driver for success while time and resources were deterrents. Incentives included altruism, professional development, and future financial opportunities.
Conclusion
The CHEER research network has significant capability and infrastructure to collect prospective data in a practice-based environment. Research coordinator engagement undergirds network success; however, future efforts will cultivate stronger collaboration of the coordinator and site PI. Central coordination of practice-based research through a hub and spoke concept can be successful.
Keywords: tinnitus, dizziness, research network, data collection, practice-based research
INTRODUCTION
The development of an effective partnership between community and academic clinical practices is critically important to allow the broad medical community to perform clinical and translational research, address important clinical questions, provide information about comparative effectiveness of treatment, and identify health disparities in research.1 The National Institutes of Health Roadmap initiatives include grant mechanisms designed to facilitate and enhance academic and community partnerships through development of research infrastructure for practice-based research networks (PBRNs) and community-based participatory research networks.
As these networks and efforts have emerged, our understanding of the critical elements necessary for success has been elucidated. A commentary in the Journal of the American Medical Association by Jones and Wells delineated guiding principles for success that can facilitate work with community partners.2 These important principles include: appropriate empowerment and leadership; clear and written goals, roles, privileges, and rules of engagement; ample communication and training; transparency in project activities, methods, and concepts; financial or in-kind resources for community partners; and transparent and equal standards for performance. Because a research network is a collaboration of people, these principles become the “rules of engagement” for research initiatives and authentic cooperation.
PBRNs first developed in the primary care specialties where the important and relevant questions that needed to be answered were fundamentally different from those that the academic-based randomized clinical trial was able to address. Misalignment of efficacy results from the randomized clinical trial with effectiveness and feasibility can become a dilemma when clinical research is not translated into practice. The appeal of a PBRN is that it can translate good research (enhance understanding) and solve the problem of the application of research results (enhance active adoption of new methods of disease management).
Development of an infrastructure for a PBRN presents unique challenges, due to the high degree of variability among community practices, encompassing differences in knowledge and expertise, skill level, and practice diversity. This is different from research infrastructure in academic medical centers conducting randomized clinical trials. Ideally, a PBRN with an academic medical center backbone can provide the framework for research in our communities, enhance regulatory oversight, decrease research education disparities, and provide the necessary blend of research methodology that can be more successfully integrated into active community practices.
In 2006, the National Institute on Deafness and Other Communication Disorders funded the development of our model of research infrastructure for a PBRN focused on hearing and communication disorders (DC-07-002). This network called CHEER (Creating Healthcare Excellence through Education and Research) is based upon a conceptual model of academic and community partnerships in otolaryngology. In addition to the research support of the Duke Clinical Research Institute (DCRI) (Durham, NC), CHEER utilizes other academic medical centers and professional ties as key elements of its research infrastructure.3 The relevance and cohesion of the CHEER network is increased by a strategic partnership with the American Academy of Otolaryngology, Head and Neck Surgery Foundation (AAOHNSF). This collaboration provides a greater stability and context in the physician’s professional landscape and has been successfully utilized by other practice-based networks in the medical and surgical specialties.4,5
The mission of the network is to become the national resource for practice-based clinical research in hearing and communicative sciences. In practical terms, CHEER provides the necessary infrastructure to accelerate clinical research in order to improve patient outcomes. The focus of CHEER is research education—to standardize coordinator education ‘at the ready’ to participate, patients are protected and safe, and the integrity of gathered data is assured and maintained.
In order to examine whether the infrastructure was operational and provide proof of concept for the CHEER model, the Otology Data Collection (ODC) exercise was developed with the goal of describing and measuring the network’s real capabilities and to collect data on a key population of patients that could be used as pilot data for future research initiatives.
We hypothesized that our strategic development of research infrastructure based upon a collaborative CHEER network model would provide the framework to rapidly collect epidemiologic data on patients presenting to a CHEER-affiliated practice with hearing and balance complaints.
METHODS
Network Development and Key Role of the Research Coordinator
The CHEER network is based on the National Institutes of Health Roadmap initiative to develop and test alternative models for conducting research. The network is a hub and spoke model, leveraging the academic research expertise of the hubs with the broader context of the spoke (or community) sites, ultimately aimed at achieving a diverse population for generalizable and expediently translatable results. All of the academic hubs serve in strategic thought leadership and mentorship roles, and 4 of 5 are also partnered with CHEER community sites (See appendix).
The community site principal investigator is the designated local otolaryngologist or otologist. Community site principal investigators involved with the CHEER network designated a research coordinator by identifying the individual in the practice who might best fit this need and had interest in the role. The qualifications and credentials of each coordinator were logged into the CHEER Web site through a privacy-protected portal.
The DCRI serves as the coordinating center for the CHEER network.
The experience and capacity of the network varies and is a critical element to monitor to ensure that there is appropriate project diversity and that educational needs and project complexity are geared toward capabilities. Table 1 summarizes some of the key attributes of the network sites.
Table 1.
Key investigator characteristics
| Key Characteristics* | |
|---|---|
| Years in practice | |
| 0–5 | 35.5 |
| 6–10 | 12.9 |
| 11–15 | 6.5 |
| 16+ | 45.2 |
| Sex | |
| Female | 12.9 |
| Male | 87.1 |
| Race | |
| White | 80.6 |
| Asian | 16.1 |
| Other | 3.2 |
| Practice specialty | |
| General otolaryngology | 51.6 |
| Otology/Neurotology | 48.4 |
| Hearing and auditory | 16.1 |
| Pediatric otolaryngology | 19.4 |
| Vertigo and balance | 29.0 |
| Other | 6.5 |
| Age groups seen in practice | |
| Pediatrics | 86.7 |
| Adolescent | 86.7 |
| Adults | 93.3 |
| Geriatric | 80.0 |
| Clinical research experience | |
| New to research | 26.7 |
| 1–5 years | 33.3 |
| 5+ years | 40.0 |
| Investigator has training in† | |
| Protocol/study review | 51.9 |
| ICH/GCP | 25.9 |
| Adverse events | 44.4 |
| Specimen management | 25.9 |
| Top 5 participation concerns | |
| Project participation must be voluntary | 93.1 |
| Participation must enhance professional development | 86.2 |
| Adequately understand research liabilities | 73.3 |
| Ability to separate standard of care from research costs | 51.7 |
| Disruption to patient flow | 37.9 |
| Top 3 participation incentives | |
| Altruism (patient benefit; medical knowledge) | 100.0 |
| Reimbursement for study exams | 76.7 |
| Access to state of the art protocols | 76.7 |
At onset of CHEER.
Detailed information and breakdown of type of training is available.
Values presented as %.
GCP=good clinical practice; ICH=International Conference on Harmonisation.
Each academic site has a research coordinator with protected time to facilitate communication, education, and regulatory tasks. Although no prior research experience was needed to be a site research coordinator for CHEER, all are required to complete the CHEER educational program (or equivalent) that includes the Collaborative Institutional Training Initiative (CITI) modules6 and baseline requirements of the Duke institutional review board (IRB).7 Incentives for participation include community site alignment with a local academic medical center in research; physician-to-physician communication and collaboration with common understanding of the importance of practice-based research; the ability to raise awareness of research or evidence gaps that impact everyday practice and access to state of the art protocols; and hope of future funded research. Additionally, the site coordinators are encouraged to attend the annual CHEER Coordinators Conference in Washington, DC to extend their knowledge and experience in research and reinforce their commitment and identity as the “CHEER Coords.”
Training logs are kept up-to-date at the grant principal investigator hub to verify educational requirements for participation in the network and to facilitate cross training.7 The CHEER network and infrastructure model places high value on the quality and motivation of the site coordinator, who acts as the facilitator for conduct of research and is key in assuring regulatory standards are maintained.
In monthly teleconferences, the CHEER Coordinator Advisory Board is co-chaired by an experienced DCRI and community practice coordinator. It consists of coordinators from each of the CHEER grant academic hubs along with representative coordinators from the community sites. Staff with expertise in regulatory and financial issues and methodology also sit on the board and provide input when appropriate. The Coordinator Advisory Board has proved integral in providing practical guidance and input for CHEER educational needs and project and protocol development. When a specific project is in development, the primary responsibility of the board is to evaluate the proposed research project for feasibility within the community site, monitor progress in ongoing research, and assist the central coordinating center in identifying and solving problems in the network.
The CHEER collaborative model is depicted in Figure 1. Overall coordination and research regulation is managed by the DCRI, thought leadership and mentoring is a shared responsibility between the Duke principal investigators and the co-principal investigators at the academic hub centers. Networking, communication, and clinical and scientific community outreach are coordinated through the AAOHNSF.
Figure 1.
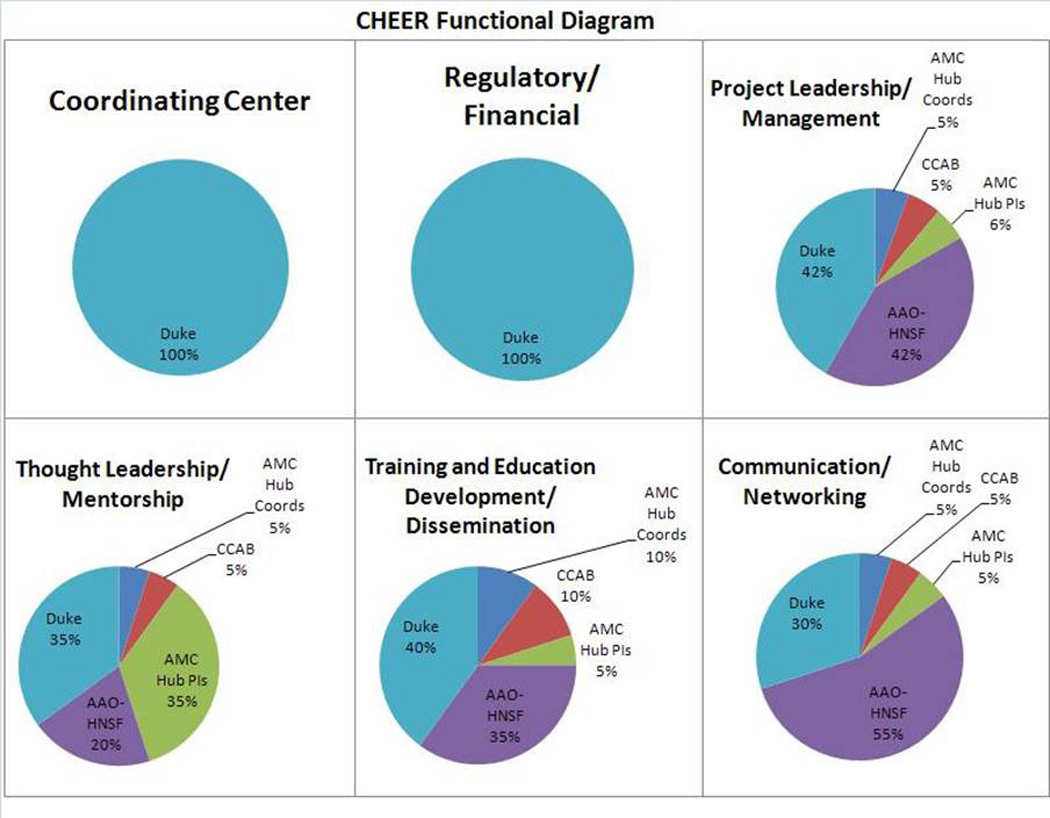
CHEER Functional Diagram
Proof of Concept—The Otology Data Collection (ODC) Pilot
The objective of the ODC pilot is to deploy a descriptive, epidemiologic exercise on 2 conditions—tinnitus and dizziness—for the purpose of proof-of-concept and testing the CHEER infrastructure and as pilot data for subsequent grant submissions.
Developed in collaboration with the CHEER principal investigator and co-principal investigators, 2 multidisciplinary expert panels were convened to identify evidence gaps and research priorities within tinnitus and dizziness that were amenable to testing within a PBRN. Based on prioritized topics, a foundational descriptive epidemiologic data collection exercise was developed and input was gathered on the draft set of questionnaires and surveys. Data collected included a set of patient questionnaires covering demographics and background information on tinnitus and dizziness as well as relevant validated questionnaires for tinnitus, dizziness, and migraine. A physician questionnaire covering clinical findings such as audiogram results, electronystagmography, and diagnosis was completed by the physician or other medical personnel. The ODC pilot exercise was vetted by the Coordinator Advisory Board for feasibility in the community setting. The introduction of the ODC data collection exercise allowed the practices to become familiar with a research data collection activity in everyday practice.
The CHEER ODC exercise is also designed to gain an estimate of the number and characteristics of patients with hearing and balance disorders that could be studied in future clinical trials seen in participating CHEER research sites. Additionally, we sought to measure the rate of patient accrual at the CHEER sites by site type.
In preparation for the ODC exercise, a site survey assessing research readiness, challenges, and IRB relationships was deployed and issues were addressed; a secure, online data capture system was developed in REDCap (Real Electronic Data Capture) for individual site access and data entry; an operational procedure training notebook was developed; an ODC message board was launched on the CHEER Web site7; and orientation conference calls were conducted. These training and deployment activities were in addition to the regular education and communication activities conducted by the CHEER Network that include an annual research coordinator training conference, quarterly newsletters, Web site updates, educational materials, and status reports.
Study data were collected and managed using REDCap electronic data capture tools hosted at the Duke University School of Nursing.8 REDCap is a secure, Web-based application designed to support data capture for research studies, providing: (1) an intuitive interface for validated data entry; (2) audit trails for tracking data manipulation and export procedures; (3) automated export procedures for seamless data downloads to common statistical packages; and (4) procedures for importing data from external sources.
The CHEER ODC was submitted to the Duke IRB for expedited review and a waiver was procured. For sites with required IRB relationships, the Duke IRB response was useful in navigating additional site IRBs. In many cases, this was the first experience for the CHEER coordinator at these sites in preparing and navigating the IRB process (with support from the CHEER team). Other than educational training and networking with colleagues, the site coordinators did not receive any financial incentive to participate in this exercise.
The initial ODC data collection exercise was designed to be 6 months in duration, at which point the data would be examined by descriptive statistics and stratified to describe individual site performance metrics. Additionally, we sought to explore the “ease” of participation, how the infrastructure worked, and where improvements should be targeted.
RESULTS
At the Annual Research Coordinator’s Conference in August 2010, a survey was conducted to get both general feedback on CHEER participation and specific feedback related to operationalizing the ODC Pilot exercise. Thirty-five percent of CHEER sites had less than 5 years of clinical experience while slightly over 45% indicated that they had over 16 years of clinical experience in their practice. There was clearly a trend for the community CHEER sites to have a focus in general otolaryngology and/or the otology subspecialty. Over half of the investigators had some experience in clinical research. The most frequent concern about participation in the project was that the project was entirely voluntary, but this concern was mitigated by the hope of future projects with funding and their commitment to the “house of medicine” or altruism. On the site coordinator side, sufficient time allocation to the project was a leading concern. The research education that is offered by CHEER was seen as a benefit of participation as well as the opportunity to network with peers. Additional comments on general participation in the network and on the ODC Pilot exercise specifically are presented in Tables 2 and 3, respectively.
Table 2.
Results of CHEER coordinators check-in survey* (n=24)
| Question | Result (%) |
|---|---|
| Who do you think is most important to your site staying committed/engaged in CHEER? | |
| Coordinator | 73.9 |
| Principal investigator | 0.0 |
| Both/equally | 26.1 |
| How engaged do you feel your PI is (select one answer that best fits)? | |
| Sees a future from CHEER and being engaged in research | 47.8 |
| Engaged/committed only if time permits | 43.5 |
| Engagement/commitment dwindling unless resource support | 8.7 |
| What are some positive benefits you, your PI, and/or your office have experienced because of CHEER (select all that apply)? | |
| Research education | 76.0 |
| Networking with other offices | 76.0 |
| Contributing to the greater good for patients | 68.0 |
| What are the greatest challenges related to CHEER (check all that apply)? | |
| Time | 68.2 |
| Setting up processes | 40.9 |
| Lack of understanding of others in the office | 31.8 |
| What are some incentives that you feel would benefit CHEER and ensure future commitment and sustainability (check all that apply)? | |
| Paid studies | 77.3 |
| CEUs for annual training | 45.5 |
| PI engagement activities | 40.9 |
| Overall, as a coordinator, how do you feel about CHEER? | |
| I enjoy it and feel I’ve made it | 65.0 |
| I enjoy it but am still trying to infuse it into our practice | 35.0 |
| I find it to be a burden on top of other responsibilities | 0.0 |
At annual research coordinators conference (August 2010).
CEU=continuing education unit; PI=principal investigator.
Table 3.
Results of CHEER ODC project survey* (n=21)
| Question | Result (%) |
|---|---|
| If you did not participate (n=6), what were the major reasons (check all that apply)? | |
| No major push/lack of interest from the coordinator | 0.0 |
| No major push/lack of interest from the principal investigator | 20.0 |
| Time | 60.0 |
| Resources | 40.0 |
| Getting staff buy-in | 0.0 |
| Participating sites (n=15) | |
| Who was the major driver to participate? | |
| Coordinator | 68.8 |
| Principal investigator | 0.0 |
| Both | 31.3 |
| Overall, was the study easier or harder to launch than anticipated? | |
| Easier | 18.8 |
| Harder | 25.0 |
| As expected | 56.3 |
| Did the CHEER network provide everything you needed in a timely fashion to facilitate participating and launching? | |
| Yes | 10.0 |
| No | 0.0 |
At annual research coordinators conference (August 2010).
Proof-of-Concept: Ability of the Network to Effectively and Expeditiously Deploy a Data Collection Exercise
A total of 1044 patients were enrolled (presenting with tinnitus, dizziness, or both) in 6 months across 15 sites or 68% of the network. Thirteen states were represented in the data collected. In terms of demographics, the average age was 54, 61% of patients were female, 63% were married, 51% were employed full-time, and 88% were white. Despite targeted recruitment of diverse sites, 88% of the patients enrolled were white; strategies for recruiting a more diverse patient population through the network are underway. Feedback from coordinators with access to diverse patient populations also indicated some difficulty in recruiting diverse patients because of preconceived notions of research or potentially culturally-based reasons.
Operationally, the ODC project was launched expediently. Case report forms were finalized in November 2010, IRB review accomplished by mid-December, and the electronic database was programmed and tested in the months of January and February 2011. Nine of 16 sites (56%) were enrolling patients into the project within the first 30 days of study launch. Enrollment rates (date patient was entered into the REDCap system) (Figure 2), expressed as percent of total, over the 6 months were 20% (March), 17% (April), 11% (May), 20% (June), 22% (July), and 11% (August). Five sites (2 academic and 3 community) pulled billing records and contributed 50% of the total enrollment. Among these sites, imputed enrollment rates for the ODC project ranged from 7–25%.
Figure 2.
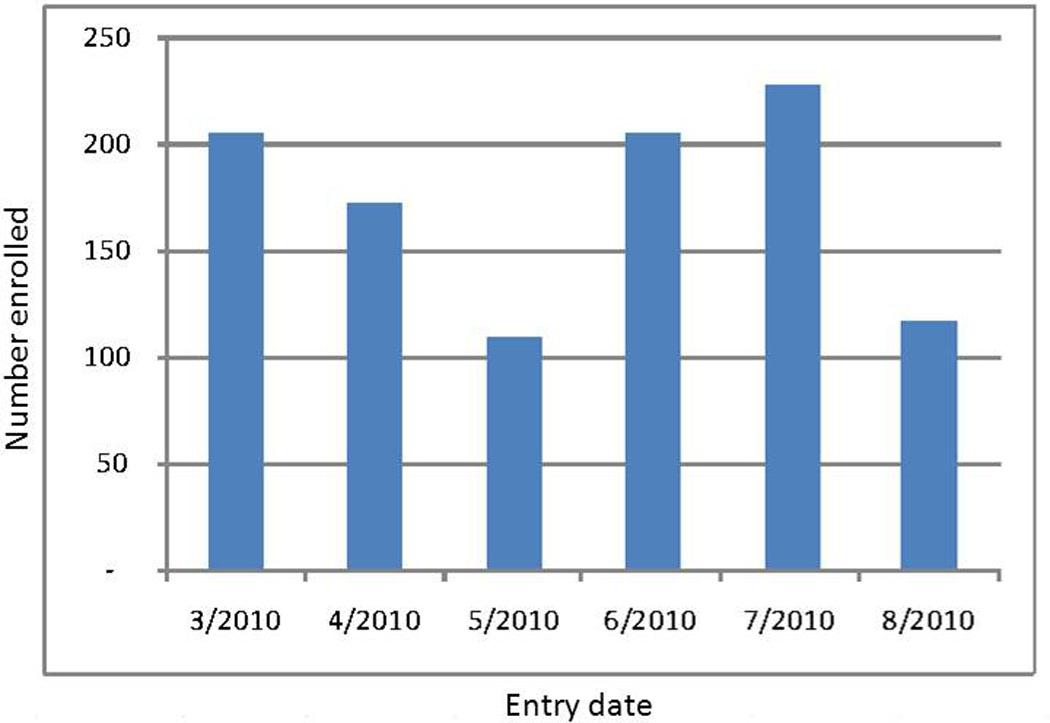
CHEER ODC Project Enrollment by Entry Date into REDCap
Interim data were used in an R21/33 submission for tinnitus phenotyping submitted in June 2010 and more up-to-date data are currently being used in the development of an R01 submission in migrainous vertigo profiling for submission in 2011. Both of these efforts continue to engage the multidisciplinary expert panels deployed prior to the ODC Pilot exercise.
DISCUSSION
We have demonstrated that the CHEER network provides an effective framework for collaborative research to permit the expedient collection of epidemiologic data from patients presenting to CHEER-affiliated practices with hearing and balance complaints. PBRNs are highly successful in collecting data and deploying projects of multiple purposes, including descriptive epidemiologic baseline data that may serve as pilot data for the development of more complex research grants and studies. Networks like CHEER are a necessary resource for the research community, both for pilot data collection and for the expedient deployment of research studies in a diverse population, facilitating research translation into practice.
Engaging coordinators in the thought leadership of a community-based research network is a crucial component of the success and viability of the network. In our model, the CHEER Coordinator Advisory Board serves in that role, with coordinators representing perspectives of all site types. When the network was polled, 74% indicated that the coordinator was the most important person on the site-based research team; a committed and educated coordinator is able to greatly facilitate all aspects of engagement in the research process. The network also recognizes the need to engage the principal investigators on a regular basis. This can be facilitated through targeted summary email updates and a Web page on the CHEER Web site with project, activity, and opportunity summaries. Additionally, more work needs to be done to access and engage a more diverse provider and patient population.
Randomized clinical trials principally examine efficacy, and that is critically important. However, efficacious treatments are not always translated into effective health care applications. A fully functional and engaged PBRN, such as CHEER, can best examine the effectiveness of a new or disputed intervention as it is implemented across health care providers.
The model that CHEER represents is an important step in this direction. We have incorporated the experience of other successful PBRNs and the experience of previous efforts at community-based research in otolaryngology3,9–11 into a functional network of data collaboration in this epidemiologic project. Research education, thought leadership, and research regulatory oversight are the foundation upon which the project was built. Our purpose in creating the CHEER research infrastructure was that a project launched from a solid foundation of committed health care workers with a common knowledge base and vision will result in better research. In our opinion, this is true of the ODC project. While the simplicity of the ODC project is noted, the magnitude of the success is noteworthy and creates a platform to build, in a stepwise fashion, a capacity to bring research into practice for treatment comparisons and outcome assessments.
The future of CHEER is embodied in its acronym, cheery. There are challenges ahead and complexities to address but the cooperation that we have seen from our sites speaks to the urgency of the need for highly relevant clinical research and the willingness of practitioners to invest in the future. As CHEER develops, the network will need to develop a full and robust portfolio of important and viable research initiatives that are woven into the fabric and culture of health care delivery for patients with ear, nose, and throat diseases. Refinement of research processes, expansion of the community network and collaboration with other academic sites and investigators will need to be an ongoing priority based upon thoughtful and deliberate planning, research expertise, and capacity.
Appendix
Active CHEER Sites by Academic Hub (n=26)
Network size: There are 5 CHEER academic hubs and PIs and 21 “community” sites that are considered active, for a total of 26 sites. “Active” means the sites are responsive, participate in conference calls and the annual coordinator’s conference, submit training information, and participate/respond to requests for information.
Academic Hub Facilities and Principal Investigators
Duke University, Debara Tucci, MD
University of Michigan, Steven Telian, MD
Massachusetts Eye and Ear Infirmary, Steven Rauch, MD
University of Texas Southwestern, Peter Roland, MD
Washington University St Louis, Jay Piccirillo, MD
Duke Sites:
Charlotte ENT Associates, NC
Low Country ENT, SC
Medical University of South Carolina
House Ear Clinic, CA
Philadelphia ENT Associates, PA
University of California Irvine Medical Center
Summit Medical Group, NJ
Coastal Ear, Nose and Throat, NJ
ENT Surgeons of Western New England, MA
University of Michigan Sites:
Ear, Nose and Throat Associates PC, IN
New York Ear and Eye Infirmary
Ear Institute of Chicago, IL
University of Maryland
Puget Sound Hearing and Balance, WA
Massachusetts Eye and Ear Infirmary:
CHEER PI site—strategic thought leadership/mentoring; currently not active recruiting site
University of Texas Southwestern Sites:
Tucson ENT, AZ
Oregon Health Sciences University
University of Texas Medical Branch
Washington University Sites:
Commonwealth ENT, KY
Metro Ear, Nose and Throat Group, MO
St. Louis ENT Health, MO
Sound Health Services PC, MO
CHEER Coordinator Advisory Board (2009–2011)
Academic/CHEER Hub, Board Members:
University of Michigan, Bianca Waller, Laura Eldred
UT Southwestern Medical Center, Barbara Staves
Washington University School of Medicine, Sara Kukuljan, Joyce Nicklaus
Duke Clinical Research Institute, Cresha Cianciolo
Non-Hub Academic, Board Members:
University of Maryland, Fleesie Hubbard-Coursey
Board Members:
Community Charlotte ENT Associates SouthPark, Angela Price
Community Commonwealth ENT, Marti Gardner
Non-voting Staff Members:
AAO-HNSF, Kris Schulz
Duke Clinical Research Institute, Kathy Moore
Ex-officio:
Duke Clinical Research Institute, Jean Bolte
References
- 1.Lindbloom EJ, Ewigman BG, Hickner JM. Practice-based research networks: the laboratories of primary care research. Med Care. 2004;42:III45–III49. [PubMed] [Google Scholar]
- 2.Jones L, Wells K. Strategies for academic and clinician engagement in communityparticipatory partnered research. JAMA. 2007;297:407–410. doi: 10.1001/jama.297.4.407. [DOI] [PubMed] [Google Scholar]
- 3.Tucci DL, Schulz K, Witsell DL. Building a national research network for clinical investigations in otology and neurotology. Otol Neurotol. 2010;31:190–195. doi: 10.1097/MAO.0b013e3181c9940c. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Wasserman RC, Slora EJ, Bocian AB, et al. Pediatric research in office settings (PROS): a national practice-based research network to improve children’s health care. Pediatrics. 1998;102:1350–1357. doi: 10.1542/peds.102.6.1350. [DOI] [PubMed] [Google Scholar]
- 5.Genel M, Dobs A. Translating clinical research into practice: practice-based research networks—a promising solution. J Investig Med. 2003;51:64–71. doi: 10.1136/jim-51-02-07. [DOI] [PubMed] [Google Scholar]
- 6. [Accessed November 3, 2010];CITI: Collaborative Institutional Training Initiative Web site. Available at: https://www.citiprogram.org.
- 7. [Accessed November 3, 2010];CHEER: Creating Healthcare Excellence through Education and Research Web site. Available at: https://www.cheerresearch.org.
- 8.Harris PA, Taylor R, Thielke R, et al. Research electronic data capture (REDCap)—a metadata-driven methodology and workflow process for providing translational research informatics support. J Biomed Inform. 2009;42:377–381. doi: 10.1016/j.jbi.2008.08.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Witsell DL, Orvidas LJ, Stewart MG, et al. Quality of life after tonsillectomy in adults with recurrent or chronic tonsillitis. Otolaryngol Head Neck Surg. 2008;138:S1–S8. doi: 10.1016/j.otohns.2007.08.015. [DOI] [PubMed] [Google Scholar]
- 10.Goldstein NA, Stewart MG, Witsell DL, et al. Quality of life after tonsillectomy in children with recurrent tonsillitis. Otolaryngol Head Neck Surg. 2008;138:S9–S16. doi: 10.1016/j.otohns.2006.12.029. [DOI] [PubMed] [Google Scholar]
- 11.Stewart MG. Outcomes research: an overview. ORL J Otorhinolaryngol Relat Spec. 2004;66:163–166. doi: 10.1159/000079872. [DOI] [PubMed] [Google Scholar]


