Abstract
Recently, a number of chemical and drug screens using zebrafish embryos have been published. Using zebrafish dystrophin mutants, we screened a chemical library for small molecules that modulate the muscle phenotype and identified seven small molecules that influence muscle pathology in dystrophin-null zebrafish. One chemical, aminophylline, which is known to be a non-selective phosphodiesterase (PDE) inhibitor, had the greatest ability to restore normal muscle structure and to up-regulate cAMP-dependent protein kinase (PKA) in treated dystrophin deficient fish. Our methodologies, which combine drug screening with assessment of the chemical effects by genotyping and staining with anti-dystrophin, provide a powerful means to identify template structures potentially relevant to the development of novel human muscular dystrophies therapeutics.
Introduction
Zebrafish quickly produce large numbers of mutant offspring that can be assayed in multiwell plates, and treated with chemicals to determine if disease progression is affected. Many of these platforms have been highly successful to model human disease and screen drugs [1–6], making the zebrafish ideal for high-throughput whole-organism screening of candidate compounds. Zebrafish also represent a validated model to investigate the function of genes that underlie human muscular dystrophy [7–19]. The orthologs of many components of the dystrophin-glycoprotein complex (DGC) s are expressed in zebrafish [20]. Our laboratory has two available fish models of Duchenne muscular dystrophy (DMD) that are dystrophin deficient, i.e. the sapje [7,9] and the sapje-like fish [10]. Dystrophin deficiency in fish results in a muscle pathology that can be detected by birefringence early in development under polarizing light. In this article, we describe the methodologies that we optimized to carry out drug screening, as well as present the candidate chemicals that restored normal muscle structure in the dystrophin-null fish.
Fish models of muscular dystrophy
Examples of fish models of human muscular diseases are listed in Table 1. These fish models have muscle phenotypes that can be detected with the birefringence assay, and include sapje [7,9], sapje-like [10], candyfloss, laminin alpha 2 mutant fish [18], dystroglycan null fish [19], and runzel, titin mutant fish [17]. In our laboratory, the two fish models of DMD used for chemical screening were the sapje (stop codon in exon 4) and sapje-like (splice site mutation in exon 62) mutants. Most of these mutant fish die before reaching adulthood. In parallel to these established models, it is possible to investigategene expression, by using anti-sense morpholino oligonucleotides (MO) to knock down specific gene expression in zebrafish experiments. The effects of morpholino on gene expression lasts about 5 days. The knocked down fish morphants, which are generated by injecting MOs into the yolk of one- to two-cell stage embryos, are particularly useful to study the function and expression of target genes and proteins during early development. In studies focused on muscle diseases, various morphants have been characterized and shown to display phenotypic features similar to those found in human patients These include morphants that downregulate the Fukutin Related Protein (FKRP) [12,13], fukutin [16], dysferlin [14], delta-sarcoglycan [8], or collagen VI [15]. Both muscle disease model fish, as well as morphant fish, can be used to analyze muscle phenotypes and in chemical screens aimed at identifying compounds that can restore normal skeletal muscle.
Table 1.
Model fish of muscle diseases
| Mutant fish | Mutated gene | Related human muscle disease | References |
|---|---|---|---|
| Sapje | Dystrophin | Duchenne Muscular Dystrophy (DMD) | [7,9] |
| Sapje like | Dystrophin | DMD | [10] |
| Candyfloss | Laminin alpha2 | Congenital Muscular Dystrophy (CMD 1A) | [17] |
| Dystroglycan null fish | Dystroglycan | Dystroglycanopathies | [18] |
| Runzel | Titin | Limb Girdle Muscular Dystrophy (LGMD 2 J) | [19] |
Chemical screeening using dystrophin null fish
Zebrafish cultur
The zebrafish (Danio rerio), embryos develop quickly: within three days of fertilization, a zebrafish has skeletal muscle, a vascular system, a beating heart, and various other organs. We maintain our model fish lines in mixed groups of heterozygous and wild type fish because most homozygous mutant fish die before reaching adulthood. Once identified, the heterozygous fish are mated in order to get progeny that include 25% of mutant embryos for chemical screening. The muscle degeneration phenotype in these mutant fish is transmitted in a recessive manner such that 25% of the offspring show degenerative muscle symptoms after 3 dpf. Twenty pairs of heterozygous sapje fish, or sapje-like fish, were mated, and fertilized eggs were cultured at 28.5 °C according to standard procedures and standard criteria [21,22] under the guidelines of our Institutional Animal Care and Use Committee.
Small Molecular Library
A range of small molecule libraries is currently available from various academic facilities (e.g. Harvard ICCB) or companies. The Prestwick chemical library (Harvard ICCB) was used as the source of small molecules for this screening. This library contains 1120 small molecules, composed of 90% marketed drugs and 10% bioactive alkaloids or related substances. The active compounds in the library were selected for their high chemical and pharmacological diversity, as well as for their known bioavailability and safety in humans. Two additional libraries are available from Harvard ICCB, the ICCB Known Bioactives Library (480 chemicals) and the NINDS2 Compounds Library (1040 chemicals) library.
The screening methods
Initial screen
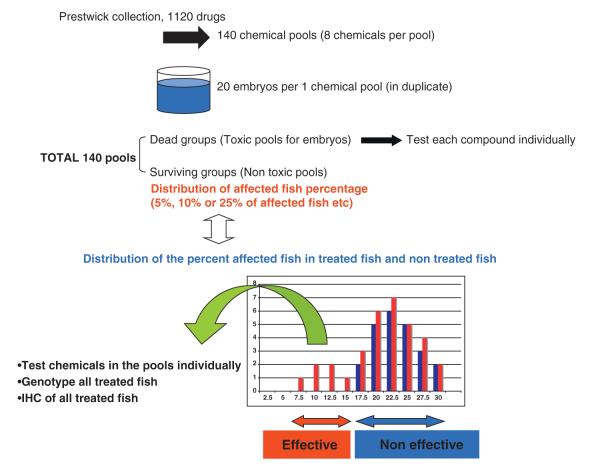
Chemicals from the Prestwick collection were combined into pooled groups of 8-compounds per pool, in duplicate, as described in Figure 1. A total of 140 chemical pools, derived from the 1120 chemicals in the library, were tested using the embryo offspring of heterozygous parents. Twenty embryos (1 dpf) per well, each containing one pool of 8 chemicals, were cultured from 1 dpf to 4 dpf in duplicate. At 4 dpf, all cultured embryos were assayed using the birefringence assay. Depending on the results of birefringence assay, the 140 chemical pools were divided into ‘non-toxic drug pools’ and ‘toxic drug pools’ (Figure 1). The chemicals comprising the ‘toxic drug pools’, i.e. that caused death of all treated embryos, were rescreened individually. The distribution of percent abnormal birefringence (i.e. affected fish) obtained using the ‘nontoxic pools’ was compared to that obtained with non-treated fish (i.e. controls). In the controls, the distribution of mutant fish showing abnormal birefringence remained close to 25%. The surviving groups of fish were divided into ‘effective drug pools’ (i.e. abnormal birefringence ≤15%) and ‘non-effective drug pools’ (i.e.abnormal birefringence > 15%) groups. Each chemical from an effective drug pool was tested individually in the second screen.
Fig. 1.
Schematic of drug screening using DMD model fish.In the first screen, twenty, one dpf-embryos from a mating of sapje heterozygous fish were arrayed, twenty per well, in 48-well plates containing 0.25 ml of fish water and eight pooled chemicals (vertical pool of eight chemicals in 96 well-plate) from the prestwick collection (total 1120 chemicals) in duplicate. The chemicals were at a final concentration of 2.4 μg/ml. As a normal control, twenty embryos were cultured in duplicate without chemicals. At 4 dpf, the birefringence of all fish was tested using a dissecting microscope. Since the muscle phenotype in these mutant fish is transmitted in a recessive manner, approximately, 25% of the offspring have the abnormal muscle birefringence phenotype after 4 dpf. After comparison of the percentage of affected fish at 4 dpf between chemical-treated fish (red bars) and non-treated fish (blue bars), chemical pools with a reduced percentage of affected fish upon birefringence assay compared to those in non-treated fish were selected for the secondary screen and were tested individually. All surviving fish, both normal and those with apparently restored muscle, were sacrificed. Heads were removed for genotyping and bodies were used for immunostaining.
Secondary screen
Pools were separated into individual chemicals, and tested by culturing 20 embryos in duplicate to identify which individual compound influenced the ratio of fish showing abnormal birefringence. All fish treated with each effective chemical (total forty 4 dpf embryos per chemical) were sacrificed and the head was separated from the body. Their heads were used for extraction of genomic DNA and subsequent genotyping. Their bodies were used to assess dystrophin expression by whole mount immunohistochemistry after fixation.
Birefringence assay
Muscle birefringence was analyzed by placing anesthesized embryos on a glass polarizing filter and then attaching a second polarizing filter onto a dissecting scope (Nikon, model SMZ1500). The top polarizing filter twisted until only the light refracting through the striated muscle was visible. Since the degree of birefringence is affected by the horizontal orientation of the fish, the fish were gently oscillated back and forth to account for differences in positioning. The detectable time of abnormal birefringence of muscle structures in mutant fish is different in each mutant. In sapje fish, candyfloss and dystroglycan null fish, it can be detected from 3 dpf to 7 dpf. On the other hand, reduced birefringence in runzel titin mutant fish can be observed at 6 dpf or 7 dpf. The abnormal disturbed muscle structures were observed as black spots or diminished birefringence. After evaluation of the muscle phenotype, these fish can recover after being returned into normal fish water, and can subsequently be cultured until adulthood.
Evaluation of chemical effects
Genotyping chemically treated fish
The genotypes of all surviving fish treated with each compound, and showing apparent corrective results in the second screen, were determined using genomic DNA extracted from the heads of individual fish (40 treated fish per chemical). The genomic DNA was used as template for PCR analysis using primer sets designed to detect either the sapje- or sapje-like mutation in the dystrophin gene. In the non-treated group of 4 dpf sapje heterozygous pair offspring, the resulting genotype percentages is 25% wild type, 50% heterozygous, and 25% homozygous for dystrophin-deficiency, as expected in a Mendelian distribution. When genotyped, every fish that had a detectable birefringence defect was confirmed to be dystrophin-null. However, treated fish showing clear, normal birefringence may have any of three possible genotypes: wildtype, heterozygous, or dystrophin-null. Some chemicals restored the muscle structure resulting in normal birefringence although genotyping indicated a dystrophin deficient homozygous mutant fish. These phenotype/genotype comparisons led to the identification of chemicals capable of correcting the muscle phenotype observed in the sapje and sapje-like fish, without restoration of dystrophin expression.
Analysis of dystrophin expression in the chemically treated fish
In zebrafish, primary muscle proteins (dystrophin, alpha- and beta- dystroglycan, laminin and myosin heavy chain) can be detected with corresponding antibodies using whole mount immunohistochemistory (IHC), which eliminates the need to section fish [13,14]. To evaluate the effects of chemical treatment, we examined dystrophin expression using an antibody directed against the c-terminal domain of dystrophin. A chemical treatment that causes exon skipping or read-through, may result in expression of normal dystrophin. However, when dystrophin is not detected in mutant fish showing normal muscle/normal birefringence after treatment, it is presumed that the chemical acts on alternative pathways that induce rescue of the muscle phenotype in mutant fish.
Long-term treatment of sapje fish with candidate chemicals
For long-term treatment of dystrophin deficient fish, mutant fish showing abnormal birefringence were identified under the dissection scope at 4 dpf and placed in a new plate to be treated with candidate chemicals from 4 dpf to 30 dpf. Ten affected and ten unaffected embryos were arrayed in 24 well-plates and cultured in 1 ml of fish water containing individual chemicals at 28.5 °C starting at 4 dpf in duplicate. At 5 dpf, treated fish were cultured at room temperature in 100 ml of fish water containing individual chemicals (2.5 μg/ml) until 30 dpf at room temperature. The number of surviving fish was counted and recorded every other day, and at 30 dpf their genotypes were determined followed by analysis of dystrophin expression and other protein expression using western blot and immunostaining.
Therapeutic drugs for dystrophin null fish
In the sapje fish drug screens [6], 108 pools resulted in surviving fish (32 pools caused death of all treated embryos). Among the 108 groups, some contained pools of chemicals that appeared to influence the ratio of affected fish relative to unaffected fish. In the experimental groups treated with the chemical pools, the distribution of the affected fish ranged between 2.5% to 27.5% compared to a range of 17.5% to 27.5% in the non-treated fish utilized as controls. Six groups had less than 7.5% of mutant fish showing abnormal birefringence and the corresponding compounds were selected as candidate therapeutics for restoring normal muscle function. The 256 chemicals comprising the 32 pools that caused death of all treated embryos were re-screened individually. However, among those, no chemical resulted in less than 7.5% mutant fish showing abnormal birefringence. In the second screen, seven chemicals influenced muscle structure by decreasing the percentage of affected fish using both sapje and sapje-like fish (Table 2). In the non-treated group of 4 dpf sapje heterozygous pair offspring, the percentages of resulting genotypes were 30% wild type, 45% heterozygous, and 25% homozygous for dystrophin-deficiency. All unaffected fish were confirmed to be wild type or heterozygous fish. In the treated groups, some homozygous dystrophin-null fish had normal birefringence and thus were considered phenotypically unaffected. Similar results were obtained in sapje-like fish. Immunostaining of individual fish bodies with antidystrophin antibodies indicated that homozygous dystrophin-null fish that displayed normal birefringence did not express dystrophin. Chemical treatment can thus restore normal birefringence in dystrophin null fish, without restoring dystrophin expression. To determine if treatment with a single compound could reverse the skeletal muscle phenotype once it is already present, affected fish (selected at 4 dpf by birefringence assay) were treated with each of the seven candidate chemicals identified in the screen, from 4 dpf to 30 dpf. Fish treated with one of the seven candidate chemicals, aminophylline, were able to survive longer (average lifespan 5.33 days, n=3) compared to non-treated dystrophin null fish (average lifespan 2.67 days, n=3) (Table 2). Histological and immunostaining analysis of aminophylline treated fish showed that the surviving fish had skeletal muscle structure restored to that similar to those of wild type fish, although they did not express dystrophin [6]. Aminophylline is known to be a non-selective PDE inhibitor that increases the levels of intracellular cAMP, causing activation of cAMP-dependent Protein Kinase (PKA) [6]. In aminophylline treated fish, increased levels of phosphorylated PKA were detected compared to those in wild type, and untreated dystrophin-null fish. These results suggest that intracellular cAMP is increased with aminophylline treatment.
Table 2.
Candidate chemicals for dystrophin null fish
| Candidate Chemicals | Sapje (%) | Sapje-like (%) | The average number of surviving fish at 30 dpf (n = 3,10 fish) |
|
|---|---|---|---|---|
| Epirizole | 10 | 15 | Anti-inflammatory agent | 3.67 |
| Homochlorcyclizine dihydrochloride | 10 | 12.5 | Anti-allergic agent | 0 |
| Conessine | 10 | 12.5 | Anti-allergic agent | 0 |
| Aminophylline | 7.5 | 17.5 | A non selective PDE inhibitor | 5.33 |
| Equilin | 5 | 10 | A group of steroid compounds | 2.33 |
| Pentetic acid | 7.5 | 10 | Chelating agents | 4.00 |
| Proscillaridin A | 10 | 10 | Cardiotonic agents | 0 |
Conclusions
The DMD model fish, sapje and sapje-like fish, are ideally suited for chemical screens aimed at identifying drug candidates that correct the dystrophin-deficiency induced muscle phenotype. 1) These fish are small enough to be permeable to small molecules, and can be assayed in large numbers. 2) Their muscle phenotypes are easily detectable by a birefringence assay to score chemical effects. For quick screening, pooled compounds were used for the first screening. Using these approaches, seven individual chemicals that decreased the percentage of muscle-affected fish have been identified by our group. Combining genotyping with dystrophin immunostaining to analyze the same fish after chemical treatment, provides a powerful means to assess the effects of drugs. We have identified 7 chemicals that can prevent the onset of abnormal muscle structure in dystrophin-null fish and result in an apparently normal fish, without restoring dystrophin expression. Recent reports in the literature have shown that selected compounds are able to cause exon skipping [23] or read-through [24] in mutant dystrophin cDNA and thus rescue the expression of truncated dystrophin. However, none of the seven chemicals identified in our screen restored dystrophin expression. We hypothesize that the chemical hits identified in our screen act on alternative proteins, thus potentially unraveling novel therapeutic pathways. The muscle structure of aminophylline-treated dystrophin-null fish appeared normal at 30 dpf. These results indicate that action of these chemicals may be able to restore dystrophin null muscle to normal muscle, without restoring dystrophin expression. Aminophylline has numerous anti-inflammatory effects, including the inhibition of inflammatory mediators [25–28]. Notably, other groups have previously reported that a PDE5 inhibitor restores mdx mouse dystrophic muscle to normal muscle [21,29,30]. It is known that PDE inhibitors cause an increase in intracellular cAMP and/or cGMP. One of the pathways which are up-regulated by elevated cAMP levels is the PKA pathway [31]. Up-regulation of expression of target proteins following PKA activation might be able to modulate the progression of diseased phenotype in skeletal muscle. Our screening approaches can be utilized to screen numerous drug collections to identify candidate drugs for the treatment of muscular dystrophies. The analysis of the molecular mechanisms downstream of these drugs may unravel common pathways as well as pathways specific to each compound, thus facilitating the identification of novel targets and/or the development of novel therapeutics to treat human muscle disease.
Acknowledgements
This work was supported by a grant from NINDS # 5P50NS040828-09, and the Muscular Dystrophy Association. Sequencing was performed in the IDDRC Molecular Genetics Core Facility supported by NICHD # 2P30HD018655-26
Footnotes
Conflict of interest statement There are no conflicts of interest in regard to this manuscript for any of the authors.
References
- 1.Dooley K, Zon LI. Zebrafish: a model system for the study of human disease. Curr Opin Genet Dev. 2000;10:252–256. doi: 10.1016/s0959-437x(00)00074-5. [DOI] [PubMed] [Google Scholar]
- 2.Zon LI, Peterson RT. In vivo drug discovery in the zebrafish. Nat Rev Drug Discov. 2005;4:35–44. doi: 10.1038/nrd1606. [DOI] [PubMed] [Google Scholar]
- 3.Pichler FB, et al. Chemical discovery and global gene expression analysis in zebrafish. Nat Biotechnol. 2003;21:879–883. doi: 10.1038/nbt852. [DOI] [PubMed] [Google Scholar]
- 4.Stern HM, et al. Small molecules that delay S phase suppress a zebrafish bmyb mutant. Nat Chem Bio. 2005;1:366–370. doi: 10.1038/nchembio749. [DOI] [PubMed] [Google Scholar]
- 5.Peterson RT, et al. Small molecule developmental screens reveal the logic and timing of vertebrate development. Proc Natl Acad Sci USA. 2000;97:12965–12969. doi: 10.1073/pnas.97.24.12965. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Kawahara G, et al. Drug screening in a zebrafish model of Duchenne muscular dystrophy. Proc Natl Acad Sci U S A. 2011;108:5331–5336. doi: 10.1073/pnas.1102116108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Bassett DI, Currie PD. The zebrafish as a model for muscular dystrophy and congenital myopathy. Hum Mol Genet. 2003;12:265–270. doi: 10.1093/hmg/ddg279. [DOI] [PubMed] [Google Scholar]
- 8.Guyon JR, et al. Delta-sarcoglycan is required for early zebrafish muscle organization. Exp Cell Res. 2005;304:105–115. doi: 10.1016/j.yexcr.2004.10.032. [DOI] [PubMed] [Google Scholar]
- 9.Guyon JR, et al. The dystrophin associated protein complex in zebrafish. Hum Mol Genet. 2003;12:601–615. [PubMed] [Google Scholar]
- 10.Guyon JR, et al. Genetic isolation and characterization of a splicing mutant of zebrafish dystrophin. Hum Mol Genet. 2009;18:202–211. doi: 10.1093/hmg/ddn337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Moore CJ, et al. Genes required for functional glycosylation of dystroglycan are conserved in zebrafish. Genomics. 2008;92:159–167. doi: 10.1016/j.ygeno.2008.05.008. [DOI] [PubMed] [Google Scholar]
- 12.Thornhill P, et al. Developmental defects in a zebrafish model for muscular dystrophies associated with the loss of fukutin-related protein (FKRP) Brain. 2008;131:1551–1561. doi: 10.1093/brain/awn078. [DOI] [PubMed] [Google Scholar]
- 13.Kawahara G, et al. Zebrafish models for human FKRP muscular dystrophies. Hum Mol Genet. 2010;19:623–633. doi: 10.1093/hmg/ddp528. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Kawahara G, et al. Characterization of zebrafish dysferlin by morpholino knockdown. Biochem Biophys Res Commun. 2011;413:358–363. doi: 10.1016/j.bbrc.2011.08.105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Lin YY, et al. Zebrafish Fukutin family proteins link the unfolded protein response with dystroglycanopathies. Hum Mol Genet. 2011;20:1763–1775. doi: 10.1093/hmg/ddr059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Telfer WR, et al. Zebrafish models of collagen VI-related myopathies. Hum Mol Genet. 2010;19:2433–2444. doi: 10.1093/hmg/ddq126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Hall TE, et al. The zebrafish candyfloss mutant implicates extracellular matrix adhesion failure in laminin alpha2-deficient congenital muscular dystrophy. Proc Natl Acad Sci U S A. 2007;104:7092–7097. doi: 10.1073/pnas.0700942104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Gupta V, et al. The zebrafish dag1 mutant: a novel genetic model for dystroglycanopathies. Hum Mol Genet. 2011;20:1712–1725. doi: 10.1093/hmg/ddr047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Steffen LS, et al. The zebrafish runzel muscular dystrophy is linked to the titin gene. Dev Biol. 2007;309:180–192. doi: 10.1016/j.ydbio.2007.06.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Steffen LS, et al. Zebrafish orthologs of human muscular dystrophy genes. BMC Genomics. 2007;8:79. doi: 10.1186/1471-2164-8-79. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Nusslein-Volhard C, Dahm R. Zebrafish: a Practical Approach. Oxford University Press; 2002. [Google Scholar]
- 22.Kimmel CB, et al. Stages of embryonic development of the zebrafish. Dev Dyn. 1995;203:253–310. doi: 10.1002/aja.1002030302. [DOI] [PubMed] [Google Scholar]
- 23.Lu QL, et al. Functional amounts of dystrophin produced by skipping the mutated exon in the mdx dystrophic mouse. Nat Med. 2003;9:1009–1014. doi: 10.1038/nm897. [DOI] [PubMed] [Google Scholar]
- 24.Welch EM, et al. PTC124 targets genetic disorders caused by nonsense mutations. Nature. 2007;447:87–91. doi: 10.1038/nature05756. [DOI] [PubMed] [Google Scholar]
- 25.Luo WJ, et al. Effects of aminophylline on cytokines and pulmonary function in patients undergoing valve replacement. Eur J Cardiothorac Surg. 2004;25:766–771. doi: 10.1016/j.ejcts.2004.02.024. [DOI] [PubMed] [Google Scholar]
- 26.Sullivan P, et al. Anti-inflammatory effects of low-dose oral theophylline in atopic asthma. Lancet. 1994;343:1006–1008. doi: 10.1016/s0140-6736(94)90127-9. [DOI] [PubMed] [Google Scholar]
- 27.Mahomed AG, et al. Anti-oxidative effects of theophylline on human neutrophils involve cyclic nucleotides and protein kinase A. Inflammation. 1998;22:545–557. doi: 10.1023/a:1022306328960. [DOI] [PubMed] [Google Scholar]
- 28.Asai A, et al. Primary Role of Functional Ischemia, Quantitative Evidence for the Two-Hit Mechanism, and Phosphodiesterase-5 Inhibitor Therapy in Mouse Muscular Dystrophy. PLoS ONE. 2007;2:e806. doi: 10.1371/journal.pone.0000806. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Kobayashi YM, et al. Sarcolemma-localized nNOS is required to maintain activity after mild exercise. Nature. 2008;456:511–515. doi: 10.1038/nature07414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Adamo CM, et al. Sildenafil reverses cardiac dysfunction in the mdx mouse model of Duchenne muscular dystrophy. Proc Natl Acad Sci USA. 2010;107:19079–19083. doi: 10.1073/pnas.1013077107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Debbie W, et al. An anchored PKA and PDE4 complex regulates subplasmalemmal cAMP dynamics. EMBO J. 2006;25:2051–2061. doi: 10.1038/sj.emboj.7601113. [DOI] [PMC free article] [PubMed] [Google Scholar]