The cost of cancer care is a topic at the center of a national discourse on fiscal responsibility and resource allocation. According to the Centers for Medicare and Medicaid Services, national health expenditures as a percentage of the U.S. gross domestic product (GDP) totaled 5% in 1965, but are expected to total 20% of GDP by the middle of this decade [1]. Although spending on cancer care comprises only 5% of the overall health care budget [2], these costs continue to rise at a pace more rapid than any other area of health care [3]. National cancer expenditures are projected to increase from $125 billion in 2010 to $173 billion in 2020 [4].
As an increasing number of expensive targeted therapies are adopted as standards of care, the average cost of treating common cancers is rising rapidly, with drugs accounting for approximately 40% of the overall cost of cancer care [1]. Another area of increasing costs is the use of diagnostic imaging. Significant annual increases in imaging have occurred across all major cancer types, and imaging costs have been rising at a faster rate than average total costs of care [5]. As a sequela of these rising expenditures, patients are shouldering an increasing proportion of the health care cost burden, often placing them under significant financial stress. Treatment-related costs have been shown to significantly increase financial burden among underinsured patients [6].
As we struggle to control rising national cancer expenditures, oncology providers are forced to examine practice patterns and their contributions to the overall health care cost burden. In 2010, Dr. Howard Brody presented a challenge to the leaders of all medical subspecialties to devise “top five” lists of costly treatments or diagnostics that lack the evidence base to support common use [7]. In response, the American Board of Internal Medicine has promoted the Choosing Wisely campaign, encouraging physicians to choose tests and treatments that are grounded in a solid evidence base. The American Society for Clinical Oncology, along with many other specialty societies, responded to the challenge and identified the top five areas for change in current oncology practice (Table 1) [8].
Table 1.
Choosing Wisely: Five Things Patients and Physicians Should Question [8]
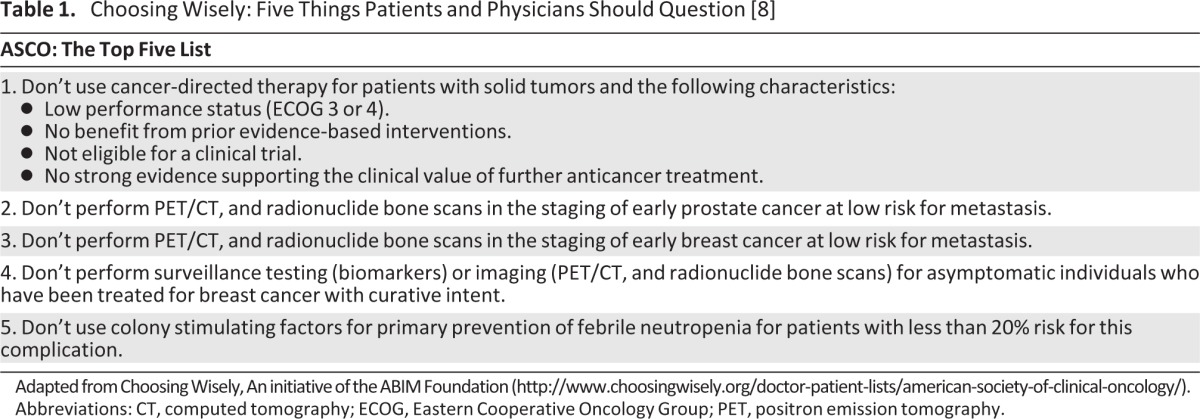
Adapted from Choosing Wisely, An initiative of the ABIM Foundation (http://www.choosingwisely.org/doctor-patient-lists/american-society-of-clinical-oncology/).
Abbreviations: CT, computed tomography; ECOG, Eastern Cooperative Oncology Group; PET, positron emission tomography.
Although this initiative represents a substantial preliminary effort, the scope of the problem is more complex. In oncology, the real problem arises when there are not enough funds to pay for all treatments and tests supported by evidence. Even among all available evidence-based treatment options approved by the U.S. Food and Drug Administration, we may be forced to prioritize the use of expensive interventions, depriving some potential candidates of access to approved medications or procedures. Given the current climate of cost-consciousness in health care, there is a pressing need to be critical of the added value of each test or treatment in order to arrive at an equitable basis for decision-making in oncology.
These issues add to the complexity of decisions that oncologists face daily at the level of the individual patient, often without a defined algorithm to guide the process. The added responsibility of considering the impact of each treatment or testing decision on the societal cost of cancer care brings up several ethical considerations. As oncologists, we find ourselves asking: is our duty to our individual patients, to society, or to both? How will we do our part to contain health care costs while honoring therapeutic contracts and professional obligations to do the best for each patient? How will the increasing pressure to curb expenditures affect the way that oncologists communicate with patients about tests and treatments?
Is Our Duty to the Patient or to Society?
An ethical conflict arises when one feels that the interests of the patient are at odds with the interests of society [9]. Oncologists are bound by duty to patients as stated in the Hippocratic Oath: “I will prescribe regimens for the good of my patients according to my ability and my judgment and never do harm to anyone.” Conflict arises when care delivered to an individual patient is part of a pattern that risks harm to society. In this case, the societal “harm”—or more accurately, burden—is in the form of skyrocketing costs of care. The conflict may be stated in the reverse: it arises when the interests of society conflict with those of the individual patient.
Some would argue that the primacy of patient welfare dictates that a physician's principal fiduciary duty is to his or her individual patient and to act in the best interests of that patient, setting aside societal concerns. In some clinical scenarios—curative intent or adjuvant therapies that have been shown to provide clear benefit—these decisions are clear-cut, and oncologists can agree that prescribing evidence-based, standard treatment is ethically mandated. Similarly, a recommendation against using diagnostic tests or treatments is not ethically fraught when such interventions have no proven benefit and may add risks. For example, oncologists are often asked by their patients with early-stage breast cancer for periodic scans and tumor markers in hopes of detecting metastatic disease before symptoms arise. Randomized studies show that routine surveillance for metastatic disease does not prolong survival or improve health-related quality of life [10–12], may lead to unnecessary or invasive testing, and contributes significantly to the cost of follow-up care [13, 14]. As a rule, physicians should not feel compelled to participate in expensive care that is not rooted in medical evidence.
However, in some situations, the decision making becomes more complex; that is, novel therapies may provide a marginal benefit, but at a high cost. For example, in the case of HER2-positive breast cancer, data in the neoadjuvant and metastatic settings show that additional HER2-based therapies given with trastuzumab, such as lapatinib and pertuzumab, may further improve outcomes [15, 16]. Most recently, trastuzumab-DM1 was approved for the treatment of metastatic HER2-positive breast cancer, and clinical trials in the adjuvant setting are planned [17, 18]. If additional benefit of these drugs is confirmed in large adjuvant randomized trials, one can imagine a scenario in which oncologists are forced to decide on further improving outcomes versus doubling or tripling the cost of therapy. As an example, the cost of trastuzumab-DM1 is $9,800 per month of treatment, for an additional 5.8 months of life in patients with metastatic disease. Similarly, other novel therapies, such as sipuleucel for advanced prostate cancer, are improving outcomes by several months, but in many cases, such progress comes at a substantial price [19, 20]. If therapies continue to be approved on the basis of marginal benefits in efficacy, how will oncologists decide which interventions to use and which patients to treat? What role will patient preference or shared decision-making have in an era of increased pressure to control costs? We have yet to confront this looming issue.
The shortage of generic cancer drugs has created an interesting response in which hospitals have established internal committees composed of internists, pharmacists, nurses, and ethicists to prioritize the use of methotrexate, liposomal doxorubicin, and other widely used drugs. In general, they have reserved these drugs for patients with curable disease, pediatric patients, and situations in which there were no reasonable therapeutic alternatives [21].
Shared Decision-Making: The Balance Between Evidence-Based Medicine and Patient-Centered Care?
It is thought that two parallel philosophies predominate in modern medicine: evidence-based medicine (EBM) and patient-centered care (PCC) [22]. EBM is defined as “the conscientious, explicit, and judicious use of current best evidence in making decisions about the care of individual patients [23].” PCC focuses on the patient's preferences for treatment and participation in decision making. On the surface, these two schools of thought are seemingly at odds. EBM attempts to standardize care and create clinical algorithms, whereas PCC aims to promote autonomy and the inclusion of the individual patient in medical decision making [24]. Both disciplines strive to improve quality of care and, ultimately, health outcomes.
Over the past two decades, there has been a great deal of research on shared decision making in oncology as a way to improve the quality of decisions in health care. A growing body of literature indicates that patients who participate actively in the decision-making process are more satisfied with the quality of care [25–27]. However, the evidence has not uniformly incorporated into routine oncologic care, and a minority of patients participate in a truly shared approach to decisions [28, 29].
Shared decision making in the context of the rising costs of cancer care provides unique opportunities to provide high-quality, patient-centered care while controlling the rising costs of cancer care. Patient decision aids, interventions, and tools that can take various forms (written, audiovisual, discussion-based) are used to facilitate the process of shared decision making; they have consistently been shown to improve decisional outcomes and patient satisfaction [30]. They are also thought to be effective tools to reduce waste and costs by helping patients choose evidence-based treatments that align with their goals and values [31].
Recent data show that patients with cancer want to discuss the costs of their care with their oncologists, which may provide another avenue for improving communication and decision quality [32]. In this issue of The Oncologist, Zafar et al. show that a substantial proportion of study participants reported a catastrophic financial burden, and nearly half of the patients reduced spending on basic necessities and used savings to pay for out-of-pocket medical expenses [6]. Nearly one-quarter of the participants reduced or avoided prescribed medications to save money [6]. Although half the patients discussed costs with their physicians, the majority of discussions were with patients applying for financial assistance with copayments [6]. These results imply a general disconnect between oncology providers and their patients with respect to conversations about costs of cancer care, and that rates of cost discussions are occurring at much lower rates in the general population [6].
The Affordable Care Act: Implications for Cost Control
Establishing the balance between effective and cost-efficient cancer care will become increasingly important as we move toward the creation of accountable care organizations (ACOs) and bundled payments [33], the goals of which are to consolidate and streamline medical resource utilization toward treating a particular diagnosis using a multidisciplinary approach [34]. Under payment bundling, each diagnosis will receive a flat-rate payment for inpatient and outpatient care in contrast to a fee-for-service system of payment [35]. This structure will provide financial incentives to ACOs to minimize or eliminate unnecessary testing and to choose among various procedures, treatments, and palliative measures, all of which provide some measurable benefit but as a whole are unaffordable. Our conclusion is that lay representatives and ethicists should be represented in these discussions to assure that the wishes and interests of the individual patient are preserved in the process of allocating resources.
Summary
The current financial constraints on cancer care delivery will only become more challenging as the costs of health care continue to rise. These ethical dilemmas represent the struggle to maintain high, evidence-based clinical standards while delivering efficient and effective care to the maximum number of patients. The oncology community must continue to examine delivery of care first through the lens of our duty to our patients, then to the practice environment, and finally to society at large. Provisions of the Affordable Care Act and a focus on shared decision making may help us move forward to achieve the goals of improved quality and reduced cost of cancer care.
Footnotes
Editor's Note: See the related article on pp. 381–390 of this issue.
Disclosures
The authors indicated no financial relationships.
References
- 1.Meropol NJ, Schulman KA. Cost of cancer care: Issues and implications. J Clin Oncol. 2007;25:180–186. doi: 10.1200/JCO.2006.09.6081. [DOI] [PubMed] [Google Scholar]
- 2.Tangka FK, Trogdon JG, Richardson LC, et al. Cancer treatment cost in the United States: Has the burden shifted over time? Cancer. 2010;116:3477–3484. doi: 10.1002/cncr.25150. [DOI] [PubMed] [Google Scholar]
- 3.Cancer Trends Progress Report—2011/2012 Update. [Accessed September 5, 2012]. Available at http://progressreport.cancer.gov.
- 4.Mariotto AB, Yabroff KR, Shao Y, et al. Projections of the cost of cancer care in the United States: 2010–2020. J Natl Cancer Inst. 2011;103:117–128. doi: 10.1093/jnci/djq495. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Dinan MA, Curtis LH, Hammill BG, et al. Changes in the use and costs of diagnostic imaging among Medicare beneficiaries with cancer, 1999–2006. JAMA. 2010;303:1625–1631. doi: 10.1001/jama.2010.460. [DOI] [PubMed] [Google Scholar]
- 6.Zafar SY, Peppercorn JM, Schrag D, et al. The financial toxicity of cancer treatment: A pilot study assessing out-of-pocket expenses and the insured cancer patient's experience. The Oncologist. 2013;18:381–390. doi: 10.1634/theoncologist.2012-0279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Brody H. Medicine's ethical responsibility for health care reform–The Top Five list. N Engl J Med. 2010;362:283–285. doi: 10.1056/NEJMp0911423. [DOI] [PubMed] [Google Scholar]
- 8.American Board of Internal Medicine. Choosing Wisely. [Accessed September 21, 2012]. Available at http://choosingwisely.org.
- 9.Farber NJ. Conflicts in the surgeon's duties to the patient and society. Thorac Surg Clin. 2005;15:527–532. doi: 10.1016/j.thorsurg.2005.06.011. [DOI] [PubMed] [Google Scholar]
- 10.The GIVIO Investigators. Impact of follow-up testing on survival and health-related quality of life in breast cancer patients. A multicenter randomized controlled trial. JAMA. 1994;271:1587–1592. doi: 10.1001/jama.1994.03510440047031. [DOI] [PubMed] [Google Scholar]
- 11.Rojas MP, Telaro E, Russo A, et al. Follow-up strategies for women treated for early breast cancer. Cochrane Database Syst Rev. 2005;(1):CD001768. doi: 10.1002/14651858.CD001768. [DOI] [PubMed] [Google Scholar]
- 12.Rosselli Del Turco M, Palli D, Cariddi A, et al. Intensive diagnostic follow-up after treatment of primary breast cancer. A randomized trial National Research Council Project on Breast Cancer follow-up JAMA. 1994;271:1593–1597. doi: 10.1001/jama.271.20.1593. [DOI] [PubMed] [Google Scholar]
- 13.Schapira DV. Breast cancer surveillance–A cost-effective strategy. Breast Cancer Res Treat. 1993;25:107–111. doi: 10.1007/BF00662135. [DOI] [PubMed] [Google Scholar]
- 14.Edelman MJ, Meyers FJ, Siegel D. The utility of follow-up testing after curative cancer therapy. A critical review and economic analysis. J Gen Intern Med. 1997;12:318–331. doi: 10.1046/j.1525-1497.1997.012005318.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Baselga J, Bradbury I, Eidtmann H, et al. Lapatinib with trastuzumab for HER2-positive early breast cancer (NeoALTTO): A randomised, open-label, multicentre, phase 3 trial. Lancet. 2012;379:633–640. doi: 10.1016/S0140-6736(11)61847-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Baselga J, Cortes J, Kim SB, et al. Pertuzumab plus trastuzumab plus docetaxel for metastatic breast cancer. N Engl J Med. 2012;366:109–119. doi: 10.1056/NEJMoa1113216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Verma S, Miles D, Gianni L, et al. Trastuzumab emtansine for HER2-positive advanced breast cancer. N Engl J Med. 2012;367:1783–1791. doi: 10.1056/NEJMoa1209124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.U.S. Food and Drug Administration. FDA approves new treatment for late-stage breast cancer. [Accessed February 28, 2013]. Available at http://www.fda.gov/NewsEvents/Newsroom/PressAnnouncements/ucm340704.htm.
- 19.Peppercorn J, Armstrong A, Zaas DW, et al. Rationing in urologic oncology: Lessons from sipuleucel-T for advanced prostate cancer. Urol Oncol. 2012 Feb 3; doi: 10.1016/j.urolonc.2011.12.022. [Epub ahead of print] [DOI] [PubMed] [Google Scholar]
- 20.Fojo T, Grady C. How much is life worth: Cetuximab, non-small cell lung cancer, and the $440 billion question. J Natl Cancer Inst. 2009;101:1044–1048. doi: 10.1093/jnci/djp177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Valgus J, Singer EA, Berry SR, Rathmell WK. Ethical challenges: Managing oncology drug shortages. J Oncol Pract. 2013 doi: 10.1200/JOP.2012.000779. (in press) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Keirns CC, Goold SD. Patient-centered care and preference-sensitive decision making. JAMA. 2009;302:1805–1806. doi: 10.1001/jama.2009.1550. [DOI] [PubMed] [Google Scholar]
- 23.Sackett DL, Rosenberg WM, Gray JA, et al. Evidence based medicine: What it is and what it isn't. BMJ. 1996;312:71–72. doi: 10.1136/bmj.312.7023.71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Hasnain-Wynia R. Is evidence-based medicine patient-centered and is patient-centered care evidence-based? Health Serv Res. 2006;41:1–8. doi: 10.1111/j.1475-6773.2006.00504.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Brown R, Butow P, Wilson-Genderson M, et al. Meeting the decision-making preferences of patients with breast cancer in oncology consultations: Impact on decision-related outcomes. J Clin Oncol. 2012;30:857–862. doi: 10.1200/JCO.2011.37.7952. [DOI] [PubMed] [Google Scholar]
- 26.Street RL, Jr., Voigt B. Patient participation in deciding breast cancer treatment and subsequent quality of life. Med Decis Making. 1997;17:298–306. doi: 10.1177/0272989X9701700306. [DOI] [PubMed] [Google Scholar]
- 27.Hack TF, Degner LF, Watson P, et al. Do patients benefit from participating in medical decision making? Longitudinal follow-up of women with breast cancer. Psychooncology. 2006;15:9–19. doi: 10.1002/pon.907. [DOI] [PubMed] [Google Scholar]
- 28.Bruera E, Willey JS, Palmer JL, et al. Treatment decisions for breast carcinoma: Patient preferences and physician perceptions. Cancer. 2002;94:2076–2080. doi: 10.1002/cncr.10393. [DOI] [PubMed] [Google Scholar]
- 29.Tariman JD, Berry DL, Cochrane B, et al. Preferred and actual participation roles during health care decision making in persons with cancer: A systematic review. Ann Oncol. 2010;21:1145–1151. doi: 10.1093/annonc/mdp534. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Stacey D, Bennett CL, Barry MJ, et al. Decision aids for people facing health treatment or screening decisions. Cochrane Database Syst Rev. 2011;10:CD001431. doi: 10.1002/14651858.CD001431.pub3. [DOI] [PubMed] [Google Scholar]
- 31.Oshima Lee E, Emanuel EJ. Shared decision making to improve care and reduce costs. N Engl J Med. 2013;368:6–8. doi: 10.1056/NEJMp1209500. [DOI] [PubMed] [Google Scholar]
- 32.Bullock AJ, Hofstatter EW, Yushak ML, et al. Understanding patients' attitudes toward communication about the cost of cancer care. J Oncol Pract. 2012;8:e50–e58. doi: 10.1200/JOP.2011.000418. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.The Patient Protection and Affordable Care Act. [Accessed March 19, 2013]. Available at http://www.gpo.gov/fdsys/pkg/BILLS-111hr3590enr/pdf/BILLS-111hr3590enr.pdf.
- 34.Emanuel E, Tanden N, Altman S, et al. A systemic approach to containing health care spending. N Engl J Med. 2012;367:949–954. doi: 10.1056/NEJMsb1205901. [DOI] [PubMed] [Google Scholar]
- 35.Moy B, Abernathy AP, Peppercorn JM. ASCO 2012 Educational Book. Alexandria, VA: American Society of Clinical Oncology; 2012. Core elements of the Patient Protection and Affordable Care Act and their relevance to the delivery of high-quality cancer care. [DOI] [PubMed] [Google Scholar]


