Heart disease is more common in those who do not receive bevacizumab. Older patients who receive bevacizumab with chemotherapy have higher odds of developing a grade 3–5 toxicity compared with those who receive chemotherapy alone.
Keywords: Chemotherapy, Geriatric assessment, Bevacizumab, Drug toxicity, Health services for the aged
Learning Objectives
Compare characteristics of older patients that receive bevacizumab plus chemotherapy to those treated with chemotherapy alone for advanced NSCLC and CRC.
Compare outcomes between older patients treated with bevacizumab plus chemotherapy to chemotherapy alone for advanced NSCLC and CRC.
Describe toxicities in older patients treated with bevacizumab plus chemotherapy for advanced NSCLC and CRC.
Abstract
Background.
Bevacizumab leads to improved survival for patients with metastatic colorectal cancer (CRC) or non-small cell lung cancer (NSCLC) when added to chemotherapy. Little is known about factors associated with receipt of bevacizumab, or whether bevacizamab is associated with increased toxicity when added to chemotherapy.
Patients and Methods.
We conducted a prospective study of patients aged ≥65 years, which evaluated the association between geriatric assessment (GA) metrics and chemotherapy toxicity. We examined differences in characteristics and outcomes of patients with CRC and NSCLC cancers who received bevacizumab with chemotherapy versus chemotherapy alone.
Results.
From a total of 207 patients, 27 (13%) received bevacizumab plus chemotherapy and 180 (87%) received chemotherapy alone. Groups were similar in sociodemographic and cancer characteristics. There were no baseline differences in GA domains except that patients with heart disease were less likely to receive bevacizumab (4% vs. 26%, p = .01). Seventy-eight percent of patients who had bevacizumab had grade 3–5 toxicity compared to only 57% who received chemotherapy alone (p = .06). Patients receiving bevacizumab were more likely to develop grade 3 hypertension than those who received chemotherapy alone (15% vs. 2%, p < .01). In multivariable analysis, factors associated with grade 3 or more toxicity included: bevacizumab (OR: 2.86, p = .04), CRC (OR: 2.54, p < .01), and baseline anemia (OR: 2.58, p = .03).
Conclusion.
Heart disease was more common in those who did not receive bevacizumab. Older patients who receive bevacizumab with chemotherapy have a higher odds of developing a grade 3–5 toxicity compared with those who receive chemotherapy alone.
Implications for Practice:
Treatment with bevacizumab necessitates closer patient monitoring, careful patient selection, and a weighing of the risk/benefit ratio in all patients. Targeted therapy should be planned with the appropriate interventions required to modify toxicity from cancer treatment in older patients.
Introduction
Inhibition of tumor angiogenesis is a major focus of cancer drug development. Bevacizumab, a humanized antibody against vascular endothelial growth factor receptor (VEGFR), was the first antiangiogenic agent to be approved for advanced non-small cell lung cancer (NSCLC) (specifically for nonsquamous histology) and colorectal cancer (CRC). Despite the fact that over 50% of patients with advanced NSCLC and CRC are older than 65 years [1], the proportion of older patients included in the studies that led to FDA approval of bevacizumab for these cancers was small. For example, the mean age in the pivotal study establishing that bevacizumab improves survival for patients with colorectal cancer was 59 years (10 years younger than the mean age of colorectal cancer incidence), and only 30% of patients were 65 and older [2]. Therefore, it is difficult to apply published data regarding the efficacy and safety of bevacizumab to the older population of patients with advanced NSCLC and CRC in the general community.
Studies of pooled clinical trial data have provided mixed results on the efficacy and safety of bevacizumab for older adults. One study (ECOG 4599) in lung cancer patients demonstrated that the addition of bevacizumab to chemotherapy did not lead to any survival benefit and was associated with higher toxicity in patients aged 70 and older compared with those who received chemotherapy alone [3]. Other studies, however, have demonstrated similar efficacy of bevacizumab in patient with lung cancer aged 65 and older compared with younger patients with no unexpected safety issues [4–6]. Results from pooled analyses evaluating the efficacy and safety of bevacizumab in older patients with advanced CRC have shown similar survival benefits between younger and older patients, although bevacizumab was associated with a higher incidence of arterial adverse events in patients aged 75 and older [7, 8]. These data suggest that prospective evaluation of the safety and efficacy of bevacizumab in advanced NSCLC and CRC is necessary.
In clinical trials, bevacizumab is associated with a higher bleeding risk, proteinuria, hypertension, and arterial thromboses when combined with chemotherapy [2, 7, 9]. These toxicities have limited the use of bevacizumab in clinical practice for older patients. Population-based data have shown that older patients are much less likely to receive bevacizumab. One study of patients in routine clinical practice found that only 54% of patients aged 65 and older with metastatic CRC eligible for bevacizumab received combination treatment versus 73% of younger patients (p < .001) [10]. There is a limited understanding regarding patient factors that influence decision-making for initiation of bevacizumab [11].
Comprehensive geriatric assessment (GA), a compilation of validated tools that capture domains that influence morbidity and mortality in older patients, can characterize the “functional age” of an older cancer patient and can help with decision-making for cancer treatment [12]. GA has been shown to identify factors that can influence outcome, and recent data demonstrate that factors within GA can predict the likelihood of developing chemotherapy toxicity, predict survival, and influence treatment decision making in older cancer patients [13–15]. GA may have value in identifying older patients who have the highest likelihood of benefiting from combination treatment with bevacizumab without significant additional toxicity.
Little is known about characteristics and outcomes of older patients who receive bevacizumab with chemotherapy for advanced NSCLC and CRC. To our knowledge, no published studies utilize GA to help identify characteristics associated with initiation of treatment and eventual outcomes. We therefore conducted a secondary analysis on data from a previously published multicenter prospective study evaluating whether GA can predict chemotherapy toxicity [13]. Patients who received bevacizumab with chemotherapy as well as those who received chemotherapy alone were enrolled in the prospective study. The objectives of this retrospective analysis of a substudy cohort with advanced NSCLC with nonsquamous histology and CRC (n = 207) were to examine differences in characteristics and outcomes of patients who received bevacizumab with chemotherapy versus chemotherapy alone and to examine the relationship of demographics, cancer characteristics, and GA variables on toxicity outcomes in bivariate and multivariable analyses.
Patients and Methods
The Cancer and Aging Research Group Study “Determining the Utility of an Assessment Tool for Older Adults with Cancer” was a multi-institutional trial open at seven participating institutions [13]. From November 2006 to 2009, over 600 patients were identified and recruited from the outpatient oncology practices at the participating institutions. Patients were eligible if they were aged ≥65, had a cancer diagnosis, were scheduled to receive a new chemotherapy regimen, and were fluent in English (because all measures in the assessment tool were not validated in other languages). We conducted a secondary analysis on these data to examine differences in characteristics and outcomes of patients with metastatic colorectal and lung cancers who received combination therapy (chemotherapy and bevacizumab) versus chemotherapy alone. In this analysis, patients who were eligible for bevacizumab (metastatic colorectal and lung) were evaluated (n = 207); the decision to give bevacizumab or not was at the primary team's discretion.
The study was approved by the institutional review board at each participating institution. All participating patients completed the informed consent process.
Study Schema
Before initiation of the chemotherapy regimen, a geriatric assessment tool was completed. The measures that comprise the tool are detailed in a prior publication describing the development of the tool [16]. The geriatric assessment tool was composed of a health care provider portion and a patient portion. The health care provider portion consisted of three items: rating the patient's Karnofsky performance status [17], timed “Up & Go” (a performance-based measure of functional status) [18], and Blessed Telephone Information-Memory-Concentration Test (a screening measure of cognitive function) [19]. The patient portion consisted of self-reported measures of functional status, comorbidity, medications, nutrition, psychological state, and social support/function. A member of the health care team assisted those who needed help completing the questionnaires.
The following treatment characteristics were captured: chemotherapy regimen, line of chemotherapy (first line or greater), the use of white or red blood cell growth factors, and the timing of initiation of white blood cell growth factors (primary or secondary prophylaxis). The chemotherapy dosing for the first cycle of treatment was categorized as standard or dose reduced as per the National Comprehensive Cancer Network guidelines [20].
The patient was followed from the beginning until the end of the chemotherapy course. Toxicities were captured at each clinical encounter (scheduled or emergency visits) by the research staff. Two physicians (the national study principal investigator [21] [PI] and site PI) subsequently reviewed the patient's chemotherapy course to capture grade 3–5 chemotherapy-related toxicities (grade 3 [severe], 4 [life-threatening], and 5 [fatal]) utilizing the National Cancer Institute (NCI) Common Terminology Criteria for Adverse Events (CTCAE), version 3.0 (http://ctep.cancer.gov/protocolDevelopment/electronic_applications/docs/ctcaev3.pdf). Blood values were captured as grade 3–5 toxicity if they met the criteria on the date of scheduled chemotherapy or at the time the patient was seeking attention because of chemotherapy toxicities.
Statistical Analyses
Descriptive analyses were performed to summarize patient, tumor, and treatment characteristics and geriatric assessment results. The incidences of the specific categories (hematologic and nonhematologic) and types of NCI CTCAE grade 3, 4, or 5 toxicity were calculated. Characteristics of patients who received bevacizumab plus chemotherapy were compared with those of patients who received chemotherapy alone. The incidences of the specific categories (hematologic and nonhematologic) and types of NCI CTCAE grade 3, 4, or 5 toxicity were calculated and compared between the two groups. We assessed the statistical significance of these differences using the chi-square test of proportions. Outcomes examined included grade 3–5 toxicity, hematologic toxicity, nonhematologic toxicity, hospitalizations, dose reduction (any), dose delay (any), and discontinuation of treatment. The main independent variable of interest was therapy (bevacizumab plus chemotherapy vs. chemotherapy alone). If bevacizumab plus chemotherapy was associated with an outcome in bivariate analyses (at p < .10), our plan was to conduct further analyses with these specific outcomes. In this bivariate analyses, bevacizumab plus chemotherapy was associated only with grade 3–5 toxicity (see Results). The relationships of demographics, cancer characteristics, and GA variables with grade 3–5 toxicity were examined in bivariate analyses. We included factors that had a p value of < .10 in bivariate analyses in the multivariable model. Adjusted odds ratios (AORs) and their 95% confidence intervals (95% CIs) indicate the independent association of each variable with the likelihood of the outcome, controlling for the effects of all other variables in the model. All statistical analyses were performed using SAS 9.1 (SAS Institute Inc., Cary, NC).
Results
Patient, Tumor, and Treatment Characteristics
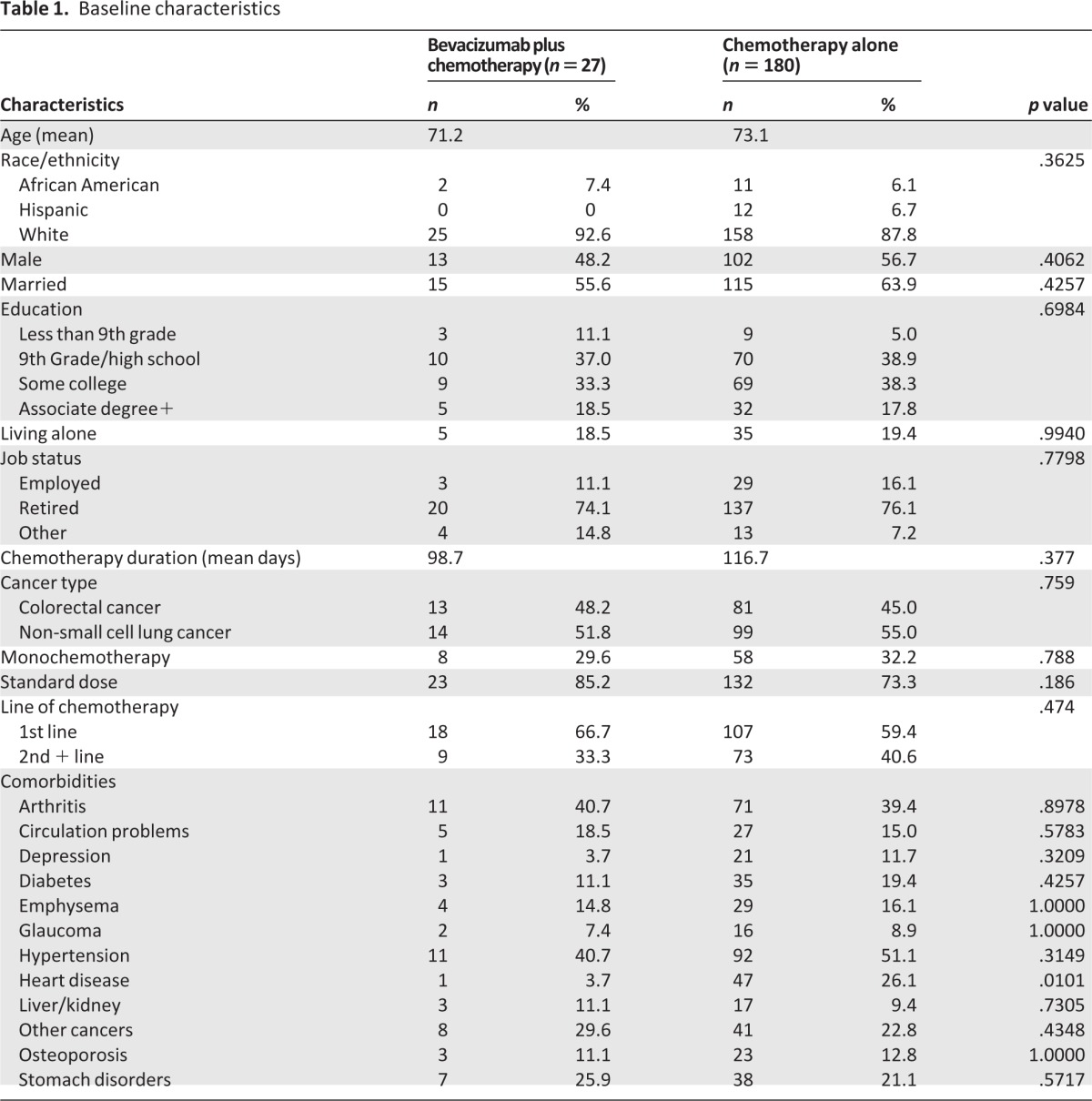
This substudy cohort consisted of 207 patients aged ≥65 with advanced stage CRC and NSCLC with nonsquamous histology (Table 1). Of the 207 patients, 180 patients received chemotherapy alone compared to 27 patients who received chemotherapy plus bevacizumab. The mean age of participants was 73 years (SD 6.1, range 65–89) and 37.7% of patients were aged 75 or older. There were no differences in age and other demographic characteristics between groups. There were no significant differences in cancer and treatment characteristics between bevacizumab plus chemotherapy and chemotherapy alone groups, 48% versus 45% had colorectal cancer and 67% versus 59% had not received previous chemotherapy. There were no baseline differences in GA domains (physical performance, falls, social support) between chemotherapy plus bevacizumab and chemotherapy alone groups except that the mean Instrumental Activities of Daily Living (IADLs) score was higher (better) in the chemotherapy and bevacizumab group compared with the chemotherapy alone group (13.2 vs. 12.5, p = .09). Comorbidities were similar in the two groups except for heart disease (26% in chemotherapy alone group vs. 4% in combination group, p = .01).
Table 1.
Baseline characteristics
Treatment Toxicity and Factors Associated with Increased Risk for Toxicity
In bivariate analyses, there were no significant associations between receipt of bevacizumab with hematologic toxicity, nonhematologic toxicity, hospitalizations, dose reduction, dose delay, and discontinuation of treatment (Table 2). Patients who received bevacizamab were more likely to have any grade 3 or greater toxicity. Seventy-eight percent of patients who received bevacizumab had grade 3 (severe), 4 (life-threatening or disabling), or 5 (death) toxicity compared to 57% who received chemotherapy alone (p = .06) (Fig. 1). More patients in the chemotherapy plus bevacizumab group developed grade 3 hypertension (15% vs. 2%, p < .01). There was no significant difference in other specific toxicities, including neutropenia and thrombosis (Table 3). The one grade 5 toxicity in the bevacizumab plus chemotherapy group was caused by GI perforation. Four grade 5 toxicities were attributed to treatment in the chemotherapy alone group, and these were a result of infection (2), liver dysfunction (1), and encephalopathy (1).
Table 2.
Association of treatment with outcomes in bivariate analyses
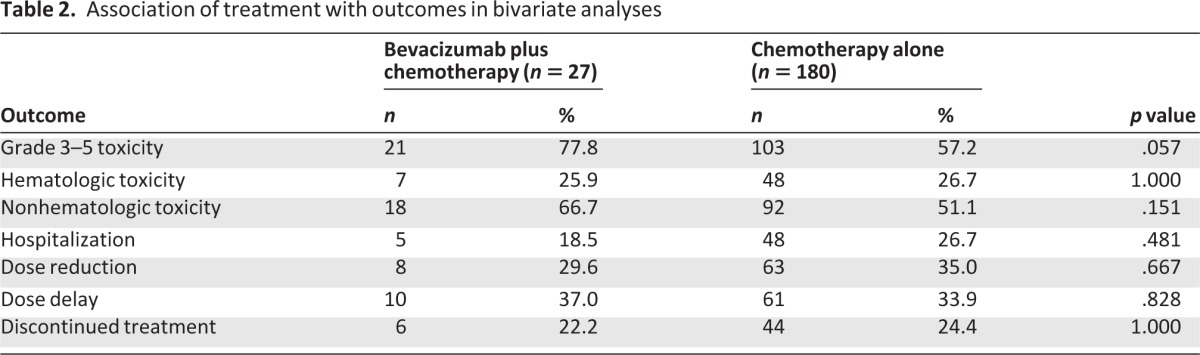
Figure 1.
Grade 3 or greater toxicity: black, chemotherapy plus bevacizumab; stripes, chemotherapy alone.
Table 3.
Summary of grade 3–5 toxicities
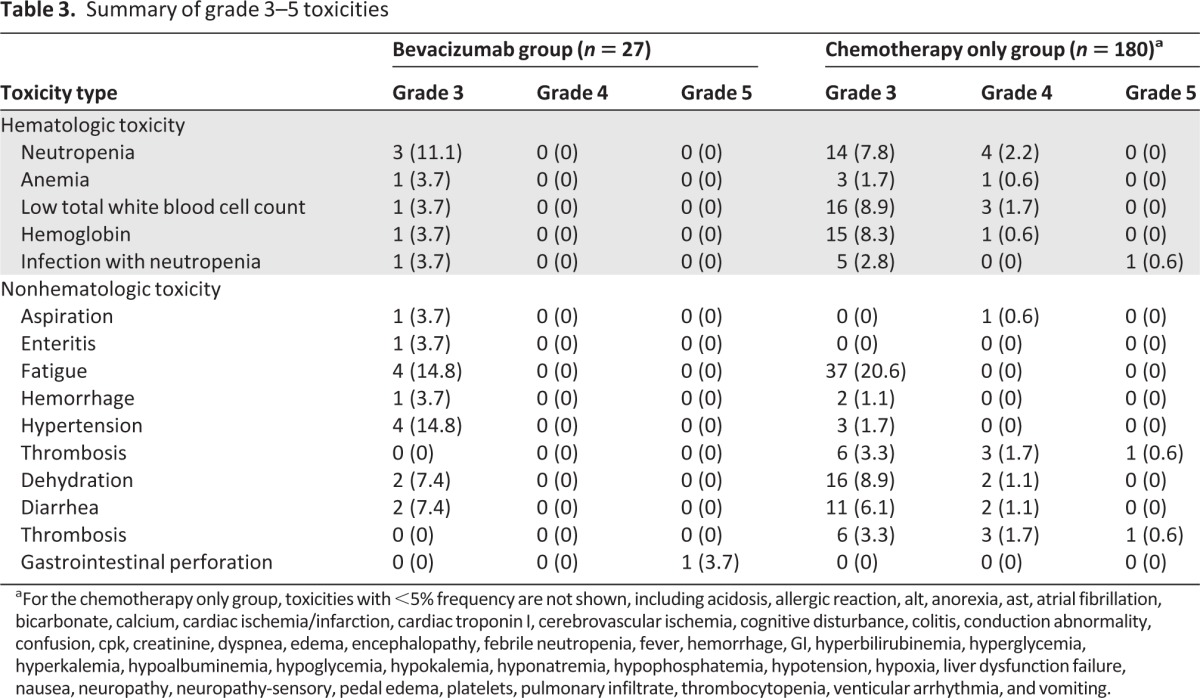
aFor the chemotherapy only group, toxicities with <5% frequency are not shown, including acidosis, allergic reaction, alt, anorexia, ast, atrial fibrillation, bicarbonate, calcium, cardiac ischemia/infarction, cardiac troponin I, cerebrovascular ischemia, cognitive disturbance, colitis, conduction abnormality, confusion, cpk, creatinine, dyspnea, edema, encephalopathy, febrile neutropenia, fever, hemorrhage, GI, hyperbilirubinemia, hyperglycemia, hyperkalemia, hypoalbuminemia, hypoglycemia, hypokalemia, hyponatremia, hypophosphatemia, hypotension, hypoxia, liver dysfunction failure, nausea, neuropathy, neuropathy-sensory, pedal edema, platelets, pulmonary infiltrate, thrombocytopenia, venticular arrhythmia, and vomiting.
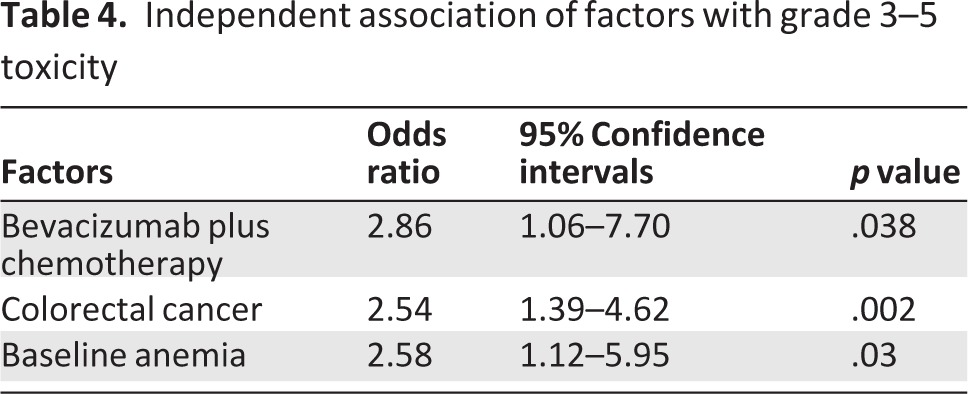
Bivariate analyses were conducted that examined the association of cancer characteristics, patient characteristics, and GA variables with grade 3–5 toxicity. Age was not associated with toxicity when evaluated as a continuous variable (p = .20). Two variables in addition to use of bevacizumab were associated with outcome at the p < .10 level and were included in the multivariable analysis. Having CRC (54.8% vs. 45.2%, p < .001) and baseline anemia (24.0% vs. 11.0%, p = .02) were associated with grade 3–5 toxicity. In multivariable analysis (Table 4), factors that remained associated with grade 3 or more toxicity included: chemotherapy plus bevacizumab (OR: 2.86, p = .04), colorectal cancer (OR: 2.54, p < .01), and baseline anemia (OR: 2.58, p = .03). No GA variables were associated with grade 3–5 toxicity in bivariate or multivariable analyses.
Table 4.
Independent association of factors with grade 3–5 toxicity
Discussion
Little is known about whether the characteristics and outcomes of older patients who receive bevacizumab for advanced CRC and NSCLC differ from those who receive chemotherapy alone in clinical practice. This study included patients with a mean age of 73, and close to 40% of patients were aged 75 and older. In this sample of older patients with advanced CRC and NSCLC, only 27 (13%) received bevacizumab plus chemotherapy compared to 180 (87%) who received chemotherapy alone. Patients who received bevacizumab plus chemotherapy had less heart disease (4% versus 26%, p = .01) and higher mean scores on functional assessment (Instrumental Activities of Daily Living, 13.2 vs. 12.5, p = .09), although the difference in IADL scores was not statistically significant. Patients receiving combination therapy were more likely to experience grade 3–5 toxicity from treatment (78% vs. 57%, p = .06), although this difference was of borderline statistical significance. Higher toxicity was, for the most part, a result of grade 3 hypertension in the bevacizumab plus chemotherapy group (15% vs. 2%, p < .01). Receipt of bevacizumab as well as having CRC were both significantly associated with higher risk for grade 3–5 toxicity in multivariable analyses. This study is the first to our knowledge to utilize GA to examine patient characteristics and outcomes that are associated with treatment with bevacizumab and chemotherapy versus chemotherapy alone.
As our population grows older, clinicians are increasingly challenged with decisions regarding treatment initiation in patients with advanced cancer. Bevacizumab was approved for first-line and second-line treatment for CRC in 2004 and 2006 and for first-line treatment for NSCLC in 2006. The mean age of participants in the pivotal studies that led to FDA approval of bevacizumab for CRC and NSCLC was around 60, which is more than 10 years younger than the average age of the population being treated [2, 9, 22]. A very small proportion of patients included in these randomized controlled trials were aged 75 and older. In addition, the studies were restricted to patients without significant health problems, which further reduces the generalizability of the data. Given the overall small benefits in survival achieved with the addition of bevacizumab, appropriate patient selection for treatment is imperative. It is clear from observational cohort studies that age does impact likelihood of receipt of bevacizumab for CRC and NSCLC [10, 23]. Differences in treatment patterns are likely related to concerns about the tolerability of these agents in patients who have characteristics (older age, comorbidities) different from those who were included in the pivotal clinical trials. Geriatric assessment, which includes a comprehensive evaluation of health status including function and comorbidities, may be helpful in identifying which patients receive standard care treatments. Our study is the first to our knowledge to examine patient selection for bevacizumab with GA and did find that patients who received bevacizumab were overall healthier than those who did not with regard to heart disease. Physicians in clinical practice are likely appropriately cautious given the toxicity profile of bevacizumab and are carefully selecting older patients for treatment with this drug. There were no other significant differences in GA metrics associated with receipt of bevacuzimab and chemotherapy.
The pivotal trials did note that severe and life-threatening toxicities were more common in patients who received bevacizumab with chemotherapy as compared with those who received chemotherapy alone, and several studies reported that these toxicities were higher in the older subset of participants. Toxicities from combination treatment include febrile neutropenia, hypertension, and thrombosis. The rate of overall grade 3–5 toxicities for bevacizumab plus chemotherapy in our study was similar to the rates reported in clinical trials, which generally report the rate of grade 3 or more toxicities to be 70% or more [2, 9, 24]. In a study of CRC patients receiving FOLFOX with bevacizumab versus FOLFOX alone, grade 4 and 5 toxicities were higher in patients aged 70 years and older [25]. In another study, a subset analysis of ECOG 4599 was conducted to evaluate the impact of age (70 and older vs. younger than 70) on toxicity from bevacizumab plus chemotherapy for patients with advanced NSCLC [3]. Grade 3 to 5 toxicities occurred in 87% of elderly patients who received treatment with bevacizumab and chemotherapy versus 61% in those who received chemotherapy alone (p < .001). Our study reports a higher rate of hypertension (15% in the bevacizumab and chemotherapy group compared to 2% in the chemotherapy alone group). Grade 3 hypertension may be able to be managed with close monitoring and antihypertensives. Our study did not show high rates of neutropenia or thrombosis in the group of patients who received bevacizumab and chemotherapy compared with those who received chemotherapy alone. These findings may be a result, in part, of careful patient selection. For example, patients who received chemotherapy with bevacizumab had less heart disease and may be less likely to have certain toxicities because of better overall fitness. One study evaluating safety of bevacizumab for older colorectal cancer patients found that use of the drug was associated with a higher prevalence of stroke, but not heart disease [26]. In addition, chemotherapy is a risk factor for thrombosis [27]. Although grade 3–5 toxicity was higher in the bevacizumab plus chemotherapy group, there was no association of bevacizumab with hospitalizations, treatment delays or dose reductions, or treatment discontinuation. Fatal toxicities reported in other studies have included GI perforation, arterial thrombosis, hypertensive crisis, and pulmonary hemorrhage. The ECOG 4599 study of older versus younger patients reported seven treatment-related deaths in the combination arm compared with two in the chemotherapy alone arm. In our study, one patient in the combination group had a fatal toxicity of GI perforation.
Our multivariable analysis illustrated that bevacizumab was independently associated with a higher likelihood of developing grade 3–5 toxicity. This result is consistent with a recent meta-analysis that illustrated that adding bevacizumab to chemotherapy was associated with an increased relative risk for fatal adverse events of 1.46 (95% CI, 1.09 to 1.94, p = .01) [28]. CRC and baseline anemia were also associated with toxicity. Several observational cohort studies are in progress to monitor the occurrence of toxicity of bevacizumab in the general practice setting and will likely provide more information regarding factors associated with toxicity. For example, ARIES is an observational cohort that includes NSCLC and CRC patients who would not have been enrolled from the pivotal phase III trial, specifically patients with poor performance status, brain metastases, and those receiving therapeutic anticoagulation. The Bevacizumab Regimens: Investigation of Treatment Effects and Safety (BriTE) Observational Cohort Study (OCS), investigating the use of first-line bevacizumab for metastatic CRC, has included patients 80 years and older, 161 (8.2%) of 1,953 patients [29]. This population is underrepresented in clinical trials, and preliminary results from BRiTE have indicated that although appropriately selected patients do benefit from bevacizumab, patients aged 75 and older had increased risk of grade 3–5 toxicities, specifically arterial thromboses.
GA factors were associated with grade 3–5 toxicity in the larger study of 500 patients, which has been previously reported [13]. This study included patients who were not receiving targeted therapies. The GA assessment revealed a number of findings that would not have been detected on routine history and physical exam including a high prevalence of patients who needed assistance with instrumental activities of daily living, had clinically significant comorbid medical conditions, were taking multiple medications, had a history of falls, and had nutritional deficits. Grade 3–5 toxicity occurred in 53% (50% grade 3, 12% grade 4, 2% grade 5). Risk factors for grade 3–5 toxicity included (a) age ≥73, (b) cancer type (GI or GU), (c) standard dose, (d) polychemotherapy, (e) falls in last 6 months, (f) assistance with instrumental activities of daily living, and (g) decreased social activity. A risk stratification scheme was developed, which was able to predict the patients most at risk for toxicity. Individual items within the GA did not predict grade 3–5 toxicities in this study, although the combination treatment (chemotherapy plus bevacizumab) was associated with grade 3–5 toxicity in multivariable analysis. This sample is a subset from the larger study [13] that included only patients who were eligible to receive bevacizumab (i.e., patients with advanced colorectal cancer or NSCLC). It may be that this population had different characteristics than the larger sample (e.g., earlier line of therapy). In addition, age was not associated with toxicity. There likely is appropriate selection of patients treated with bevacizumab and chemotherapy. Oncologists are aware that bevacizumab has a higher risk for patients with a history of heart disease, and may be especially cautious in initiating treatment for older patients with heart disease. In this study, physicians by request could have access to geriatric assessment results and may have utilized the information for selecting patients for treatment. More data on the utility of GA to predict adverse outcomes in older patients receiving targeted therapies is necessary.
There are limitations to this study. The small sample size and retrospective nature of the study limits our ability to determine cause and effect. This study reported on grade 3–5 toxicity; however, some grade 2 toxicities may also be relevant to the geriatric oncology population. Our study population was heterogeneous, consisting of patients on different treatment regimens. Another limitation is that we did not capture physician decision making regarding which factors influenced the decision to start (or not start) bevacizumab.
Our study illustrates that appropriately selected older patients may tolerate bevacizumab with chemotherapy. Only a minority of older patients in clinical practice actually receive bevacizumab with chemotherapy. Although patients who received bevacizumab were healthier, grade 3–5 toxicity was higher, and receipt of bevacizumab was independently associated with toxicity. Hypertension, the most frequent toxicity in the bevacizumab and chemotherapy group, was grade 3 in all cases and, therefore, may be able to be managed effectively with close monitoring and antihypertensives. Treatment with bevacizumab necessitates closer patient monitoring, careful patient selection, and a weighing of the risk/benefit ratio in all patients. Future studies should capture information on how physicians make decisions regarding targeted therapy for older patients and develop interventions to modify toxicity from cancer treatment in older patients.
This article is available for continuing medical education credit at CME.TheOncologist.com.
Acknowledgments
This work is supported by the Paul Beeson Career Development Award in Aging Research (K23 AG026749–01 to A.H.), and the American Society of Clinical Oncology–Association of Specialty Professors–Junior Development Award in Geriatric Oncology to A.H.
Author Contributions
Conception/Design: Supriya Mohile, Molly Hardt, William Tew, Cynthia Owusu, Heidi Kaplan, Cary P. Gross, Ajeet Gajara, Stuart Lichtman, Kayo Togawa, Rupal Ramani, Vani Katheria, Kurt Hansen, Arti Hurria
Provision of study material or patients: Supriya Mohile, Kayo Togawa, Rupal Ramani, Vani Katheria, Kurt Hansen, Arti Hurria
Collection and/or assembly of data: Supriya Mohile, William Tew, Cynthia Owusu, Heidi Kaplan, Cary P. Gross, Ajeet Gajara, Stuart Lichtman, Kayo Togawa, Rupal Ramani, Vani Katheria, Kurt Hansen, Arti Hurria
Data analysis and interpretation: Supriya Mohile, Molly Hardt, William Tew, Cynthia Owusu, Heidi Kaplan, Cary P. Gross, Ajeet Gajara, Kayo Togawa, Rupal Ramani, Vani Katheria, Kurt Hansen, Arti Hurria
Manuscript writing: Supriya Mohile, Molly Hardt, William Tew, Cynthia Owusu, Heidi Kaplan, Cary P. Gross, Ajeet Gajara, Kayo Togawa, Rupal Ramani, Vani Katheria, Kurt Hansen, Arti Hurria
Final approval of manuscript: Supriya Mohile, Molly Hardt, William Tew, Cynthia Owusu, Heidi Kaplan, Cary P. Gross, Ajeet Gajara, Stuart Lichtman, Kayo Togawa, Rupal Ramani, Vani Katheria, Kurt Hansen, Arti Hurria
Disclosures
Arti Hurria: Amgen, Genentech, Seattle Genetics, and GTx (C/A); Abraxis BioScience, Celgene, and GlaxoSmithKline (RF). The other authors indicated no financial relationships.
Section editors: Hyman B. Muss: Wyeth/Pfizer (C/A); Matti Aapro: Sanofi (C/A)
Reviewer “A”: Roche (RF)
Reviewer “B”: None
Reviewer “C”: None
(C/A) Consulting/advisory relationship; (RF) Research funding; (E) Employment; (H) Honoraria received; (OI) Ownership interests; (IP) Intellectual property rights/inventor/patent holder; (SAB) Scientific advisory board
References
- 1.Jemal A, Siegel R, Xu J, et al. Cancer statistics, 2010. CA Cancer J Clin. 2010;60:277–300. doi: 10.3322/caac.20073. [DOI] [PubMed] [Google Scholar]
- 2.Hurwitz H, Fehrenbacher L, Novotny W, et al. Bevacizumab plus irinotecan, fluorouracil, and leucovorin for metastatic colorectal cancer. N Engl J Med. 2004;350:2335–2342. doi: 10.1056/NEJMoa032691. [DOI] [PubMed] [Google Scholar]
- 3.Ramalingam SS, Dahlberg SE, Langer CJ, et al. Outcomes for elderly, advanced-stage non-small-cell lung cancer patients treated with bevacizumab in combination with carboplatin and paclitaxel: Analysis of eastern cooperative oncology group trial 4599. J Clin Oncol. 2008;26:60–65. doi: 10.1200/JCO.2007.13.1144. [DOI] [PubMed] [Google Scholar]
- 4.Leighl NB, Zatloukal P, Mezger J, et al. Efficacy and safety of bevacizumab-based therapy in elderly patients with advanced or recurrent nonsquamous non-small cell lung cancer in the phase III BO17704 study (AVAiL) J Thorac Oncol. 2010;5:1970–1976. doi: 10.1097/JTO.0b013e3181f49c22. [DOI] [PubMed] [Google Scholar]
- 5.Reck M, von Pawel J, Zatloukal P, et al. Overall survival with cisplatin–gemcitabine and bevacizumab or placebo as first-line therapy for nonsquamous non-small-cell lung cancer: Results from a randomised phase III trial (AVAiL) Ann Oncol. 2010;21:1804–1809. doi: 10.1093/annonc/mdq020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Laskin J, Crinò L, Felip E, et al. Safety and efficacy of first-line bevacizumab plus chemotherapy in elderly patients with advanced or recurrent nonsquamous non-small cell lung cancer: Safety of avastin in lung trial (MO19390) J Thorac Oncol. 2012;7:203–211. doi: 10.1097/JTO.0b013e3182370e02. [DOI] [PubMed] [Google Scholar]
- 7.Scappaticci FA, Skillings JR, Holden SN, et al. Arterial thromboembolic events in patients with metastatic carcinoma treated with chemotherapy and bevacizumab. J Natl Cancer Inst. 2007;99:1232–1239. doi: 10.1093/jnci/djm086. [DOI] [PubMed] [Google Scholar]
- 8.Kabbinavar FF, Hurwitz HI, Yi J, et al. Addition of bevacizumab to fluorouracil-based first-line treatment of metastatic colorectal cancer: Pooled analysis of cohorts of older patients from two randomized clinical trials. J Clin Oncol. 2009;27:199–205. doi: 10.1200/JCO.2008.17.7931. [DOI] [PubMed] [Google Scholar]
- 9.Sandler A, Gray R, Perry MC, et al. Paclitaxel–carboplatin alone or with bevacizumab for non–small-cell lung cancer. N Engl J Med. 2006;355:2542–2550. doi: 10.1056/NEJMoa061884. [DOI] [PubMed] [Google Scholar]
- 10.McKibbin T, Frei CR, Greene RE, et al. Disparities in utilization of irinotecan (IRI), oxaliplatin (OX), and bevacizumab (BEV) among young and elderly patients with advanced colorectal cancer (CRC). Paper presented at 2008 Gastrointestinal Cancers Symposium; 2008. [Google Scholar]
- 11.François E, Guérin O, Follana P, et al. Use of bevacizumab in elderly patients with metastatic colorectal cancer: Review. J Geriatr Oncol. 2011;2:64–71. [Google Scholar]
- 12.Rodin MB, Mohile SG. A practical approach to geriatric assessment in oncology. J Clin Oncol. 2007;25:1936–1944. doi: 10.1200/JCO.2006.10.2954. [DOI] [PubMed] [Google Scholar]
- 13.Hurria A, Togawa K, Mohile SG, et al. Predicting chemotherapy toxicity in older adults with cancer: A prospective multicenter study. J Clin Oncol. 2011;29:3457–3465. doi: 10.1200/JCO.2011.34.7625. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Kanesvaran R, Li H, Koo K-N, et al. Analysis of prognostic factors of comprehensive geriatric assessment and development of a clinical scoring system in elderly Asian patients with cancer. J Clin Oncol. 2011;29:3620–3627. doi: 10.1200/JCO.2010.32.0796. [DOI] [PubMed] [Google Scholar]
- 15.Caillet P, Canoui-Poitrine F, Vouriot J, et al. Comprehensive geriatric assessment in the decision-making process in elderly patients with cancer: ELCAPA study. J Clin Oncol. 2011;29:3636–3642. doi: 10.1200/JCO.2010.31.0664. [DOI] [PubMed] [Google Scholar]
- 16.Hurria A, Gupta S, Zauderer M, et al. Developing a cancer-specific geriatric assessment. Cancer. 2005;104:1998–2005. doi: 10.1002/cncr.21422. [DOI] [PubMed] [Google Scholar]
- 17.Karnofsky D, Burchenal J. The clinical evaluation of chemotherapeutic agents in cancer. In: MacLeod CM, editor. Evaluation of Chemotherapeutic Agents. New York: Columbia University Press; 1948. pp. 191–205. [Google Scholar]
- 18.Podsiadlo D, Richardson S. The timed “Up & Go”: A test of basic functional mobility for frail elderly persons. J Am Geriatr Soc. 1991;39:142–148. doi: 10.1111/j.1532-5415.1991.tb01616.x. [DOI] [PubMed] [Google Scholar]
- 19.Kawas C, Karagiozis H, Resau L, et al. Reliability of the Blessed Telephone Information-Memory-Concentration Test. J Geriatr Psychiatry Neurol. 1995;8:238–242. doi: 10.1177/089198879500800408. [DOI] [PubMed] [Google Scholar]
- 20.National Comprehensive Cancer Network. NCCN Clinical Practice Guidelines in Oncology. 2010. [Accessed June 13, 2012]. Available at http://www.nccn.org/professionals/physician_gls/f_guidelines.asp.
- 21.National Institutes of Health. FDA Approval for Bevacizumab—National Cancer Institute. 2009. [Accessed June 13, 2012]. Available at http://www.cancer.gov/cancertopics/druginfo/fda-bevacizumab.
- 22.Giantonio BJ. Bevacizumab in the treatment of metastatic colorectal cancer (mCRC) in second- and third-line settings. Semin Oncol. 2006;33(suppl 10):S15–S18. doi: 10.1053/j.seminoncol.2006.08.003. [DOI] [PubMed] [Google Scholar]
- 23.Bekaii-Saab T, Bendall J, Cohn A. Initial results from ARIES, a multi-indication bevacizumab (BV) observational cohort study (OCS): Characteristics of metastatic colorectal cancer (mCRC) patients (pts) receiving BV and chemotherapy (CT) in 2nd line. J Clin Oncol. 2008;26(suppl 15):15002. [Google Scholar]
- 24.Saltz LB, Clarke S, Díaz-Rubio E, et al. Bevacizumab in combination with oxaliplatin-based chemotherapy as first-line therapy in metastatic colorectal cancer: A randomized phase III study. J Clin Oncol. 2008;26:2013–2019. doi: 10.1200/JCO.2007.14.9930. [DOI] [PubMed] [Google Scholar]
- 25.Allegra CJ, Yothers G, O'Connell MJ, et al. Initial safety report of NSABP C-08: A randomized phase III study of modified FOLFOX6 with or without bevacizumab for the adjuvant treatment of patients with stage II or III colon cancer. J Clin Oncol. 2009;27:3385–3390. doi: 10.1200/JCO.2009.21.9220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Meyerhardt JA, Li L, Sanoff HK, et al. Effectiveness of bevacizumab with first-line combination chemotherapy for Medicare patients with stage IV colorectal cancer. J Clin Oncol. 2012;30:608–615. doi: 10.1200/JCO.2011.38.9650. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Sud R, Khorana AA. Cancer-associated thrombosis: Risk factors, candidate biomarkers and a risk model. Thromb Res. 2009;123(suppl 4):S18–S21. doi: 10.1016/S0049-3848(09)70137-9. [DOI] [PubMed] [Google Scholar]
- 28.Ranpura V, Hapani S, Wu S. Treatment-related mortality with bevacizumab in cancer patients: A meta-analysis. JAMA. 2011;305:487–494. doi: 10.1001/jama.2011.51. [DOI] [PubMed] [Google Scholar]
- 29.Kozloff M, Yood MU, Berlin J, et al. Clinical outcomes associated with bevacizumab-containing treatment of metastatic colorectal cancer: The BRiTE observational cohort study. The Oncologist. 2009;14:862–870. doi: 10.1634/theoncologist.2009-0071. [DOI] [PubMed] [Google Scholar]


