Figure 1. MR over-expression during neuronal differentiation.
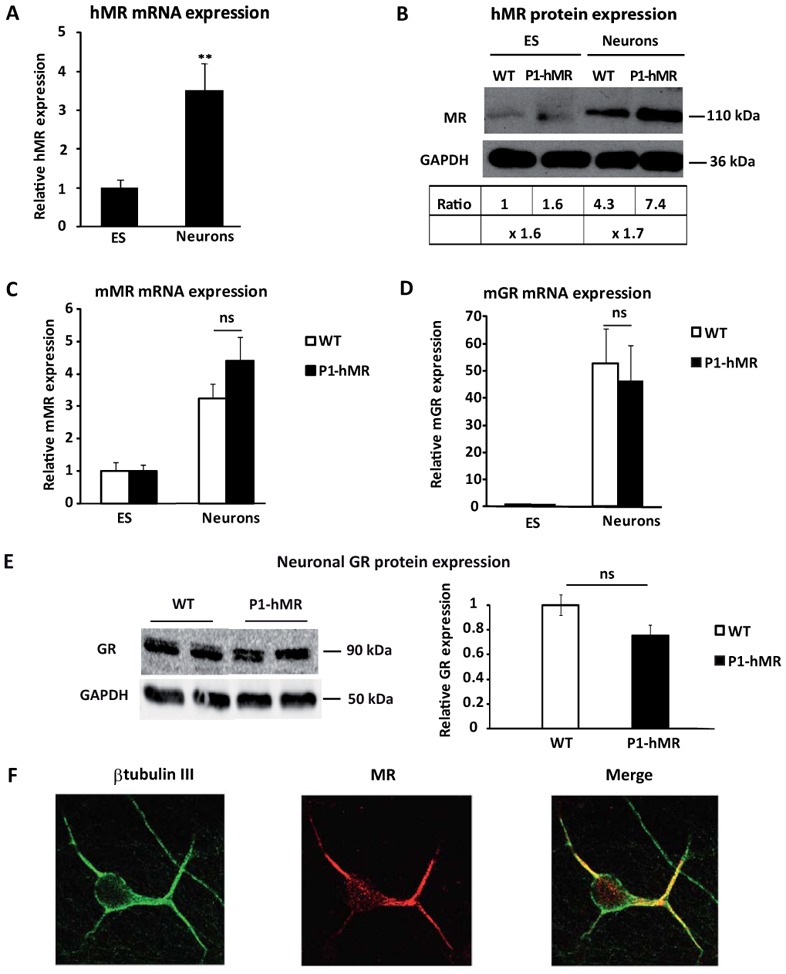
A) Relative hMR mRNA expression levels were determined using qPCR in undifferentiated ES cells and neurons. Results are means ± SEM of two independent experiments of six samples performed in duplicate for each developmental stage indicating the relative expression compared with basal levels of ES (arbitrarily set at 1). ** P<0.01. Mann Whitney test. Relative mRNA expression is normalized to 18S rRNA expression (see Materials and Methods section). B) Western blot analyses of MR protein expression in WT and P1-hMR ES cell lines. Undifferentiated ES and neurons lysates from each ES cell line were processed for immunoblotting with anti-MR antibody. GAPDH was used as loading control. MR was normalized to GAPDH protein levels after digitalization on a gel scanner with QuantityOne software (Bio-Rad, Marnes-la-Coquette, France). Results are presented as MR/GAPDH ratio and as compared with basal levels of WT ES (arbitrarily set at 1). C–D) Relative mMR and mGR mRNA expression levels were determined using qPCR in undifferentiated ES cells and neurons from WT and P1-hMR ES cell lines. Results are means ± SEM of two independent experiments on six samples performed in duplicate for each developmental stage and represent the relative expression compared with basal levels of ES (arbitrarily set at 1). Mann Whitney test. Relative mRNA expression is normalized to 18S rRNA expression (see Materials and Methods section). E) Western blot analysis of GR expression in WT and P1.hMR neurons and signal quantification of the GR/GAPDH ratio (n = 6), ns: non significant. WT mean value arbitrarily set at 1. F) Double-immunolabeling of P1-hMR neurons with antibodies against β-tubulin III (green) (left panel) and MR (red) (middle panel); merged images are shown on the right. Original magnification × 40.
