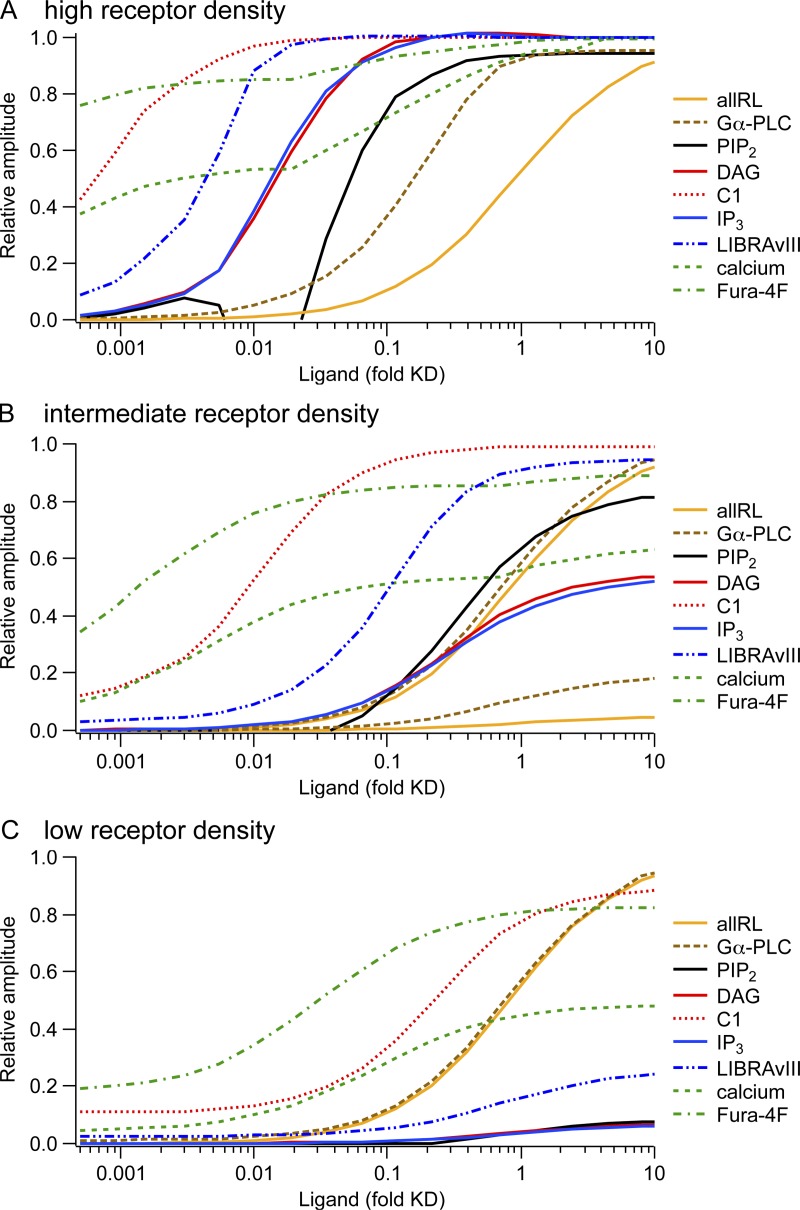
Figure 8.
Concentration–response curves from the model illustrating the concept of receptor reserve. Panels show predicted responses from the computational model for three different receptor densities (R was 500 µm−2 for A, “high density”; 25 µm−2 for B, “intermediate density;” and 1.25 for C, “low density”). The x axis represents agonist concentration in reduced units of concentration divided by Kd, the dissociation constant for receptors in the absence of G proteins. For reference, 1.0 on this axis corresponds to 2 µM Oxo-M or possibly 5 µM UTP. Curves show the responses of many signaling intermediates normalized to their extreme values (maxima or minima), with increasing species normalized to the maximum and decreasing species normalized so that 0 corresponds to the resting value and 1 corresponds to the minimum with receptor saturation. For A, PI 4-kinase and PIP2 5-kinase were accelerated during agonist as described in Fig. 7. For B, acceleration of PI 4-kinase and PIP 5-kinase was 20-fold less. For C, PI 4-kinase and PIP 5-kinase were not accelerated. The y-scaling was kept constant between A, B, and C with the following exceptions: B contains two traces “allRL” and two traces “Gα-PLC,” one of each on the same scale as in panel A to illustrate the difference in maximum amplitude and a second one of each that was rescaled to its maximum to better compare midpoints between A and B. In C, “allRL” and “Gα-PLC” are shown only rescaled to maximum. The dip in the PIP2 depletion curve between 0.005 and 0.02 agonist/Kd of A is probably an artifact of our model. It occurs because, for this concentration range, accelerated PIP2 synthesis outweighs PLC activation, leading to a net increase in PIP2. We have no direct evidence for this occurring in real cells, suggesting that the model (Fig. 7 E) introduces too much acceleration of PIP2 synthesis in this intermediate agonist concentration range.