Figure 1.
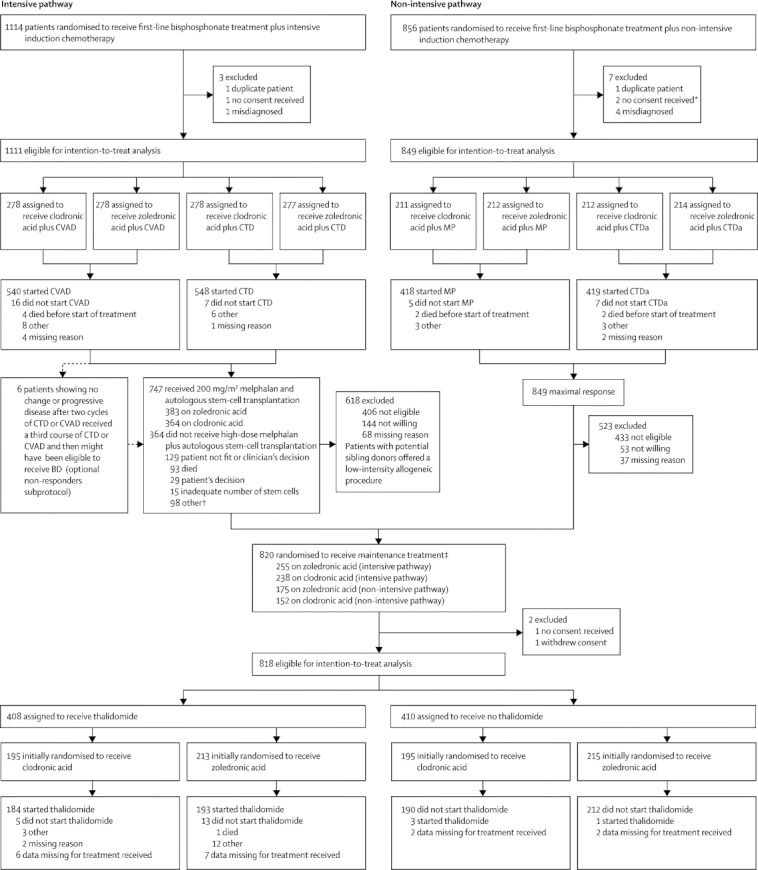
Trial profile
*Patients were included in the safety population. †Two patients on zoledronic acid received a conditioning regimen other than high-dose melphalan and underwent subsequent autologous stem-cell transplantation. ‡One patient in the non-intensive pathway who was excluded because no consent was received was then included in the randomisation to receive maintenance treatment; some of the 23 patients in the intensive pathway who did not start CVAD or CTD did receive high-dose melphalan plus autologous stem-cell transplantation, but none was included in the randomisation to receive maintenance treatment; none of the 12 patients in the non-intensive pathway who did not start MP or CTDa was included in the randomisation to receive maintenance treatment. CVAD=cyclophosphamide, vincristine, doxorubicin, and dexamethasone. CTD=cyclophosphamide, thalidomide, and dexamethasone. MP=melphalan and prednisolone. CTDa=attenuated CTD. BD=bortezomib and dexamethasone.
