Figure 5.
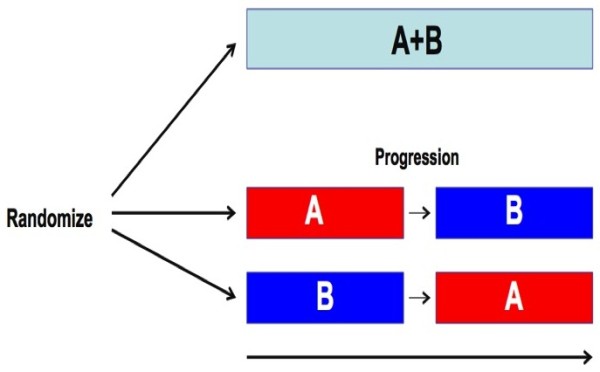
Suggested trial structure for assessment of combinations: Combination A+B would be compared to A followed at progression by B and to B followed at progression by A. Endpoints might include: 1) Time to progression on A+B vs time to progression from the initiation of the first single agent until completion of the second single agent; 2) A+B would be compared to each of A alone or B alone with respect to time to progression and maximum response; 3) Overall survival on the 3 arms; 4) Ability of B to suppress emergence of specific resistant clones while on A. 5) Of very high importance would be the development of molecular signatures that predict unique benefit of A alone, B alone, A+B combined, and the A→B/B→A sequences.
