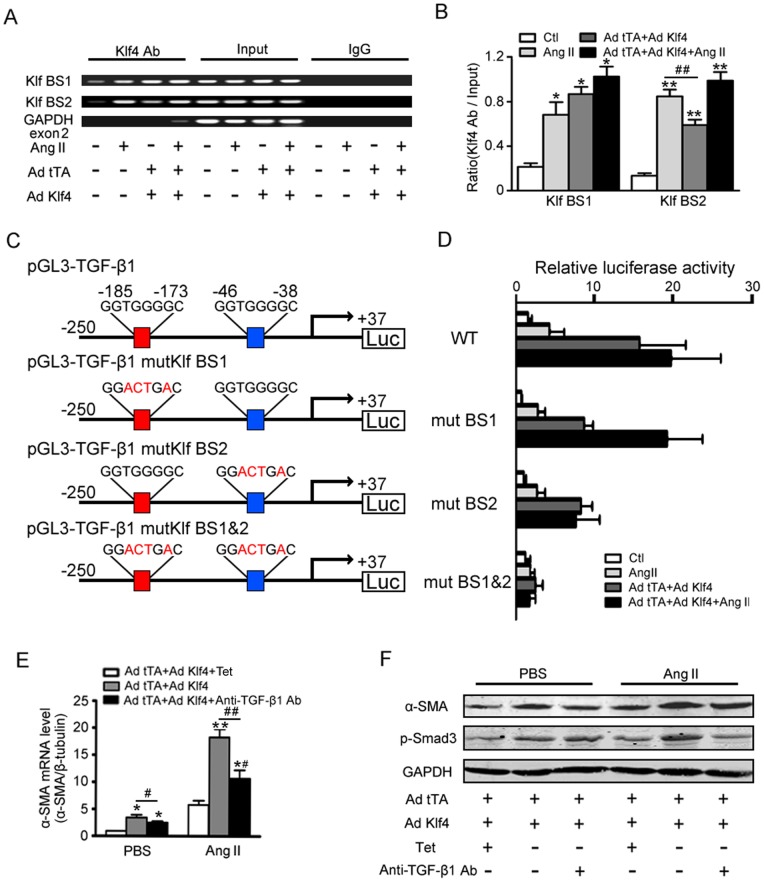
Figure 6. Klf4 transactivates the TGF-β1 promoter.
A, ChIP assays of Klf4 binding to the −184∼−180-bp site (Klf BS1) and −45∼−41-bp site (Klf BS1) of the TGF-β1 promoter. A non-target region (GAPDH exon 2) was used as a negative control. B, normalization of each ChIP DNA fraction to the input DNA fraction using densitometric units (n = 3) was shown. Data are the mean ± SEM. *P<0.05, **P<0.01 vs. Ang II untreated and Klf4 non-overexpression. ##P<0.01 vs. Ang II treatment. C, pGL3-luciferase reporter driven by the wild-type TGF-β1 promoter or mutant (at −184∼−180-bp and −45∼−41-bp promoter sites) in which the potential Klf4-binding site was constructed. D, promoter-reporter analysis for Klf4-depedent transactivation of the TGF-β1. The pGL3-TGF-β1 WT or mutant vector and CMV-renilla luciferase vector were cotransfected. Luciferase activation driven by the wild-type TGF-β1 promoter or mutant promoter and were normalized to renilla luciferase (n = 4). Data are the mean ± SEM. E-F, effects of neutralizing TGF-β1 on the cardiac fibroblast differentiation activity. An antibody against TGF-β1 (1 μg/ml) was added to the conditional medium. E, the effects of Klf4-dependent α-SMA mRNA expression was assessed by qPCR after Ang II treatment for 0 or 6 hrs (n = 4). Data are the mean ± SEM. *P<0.05, **P<0.01 vs. Klf4 non-overexpression; #P<0.05, ##P<0.01 vs. Klf4 overexpression. F, levels of α-SMA and p-Smad3 were assessed by Western blot after Ang II treatment for 24 hrs.