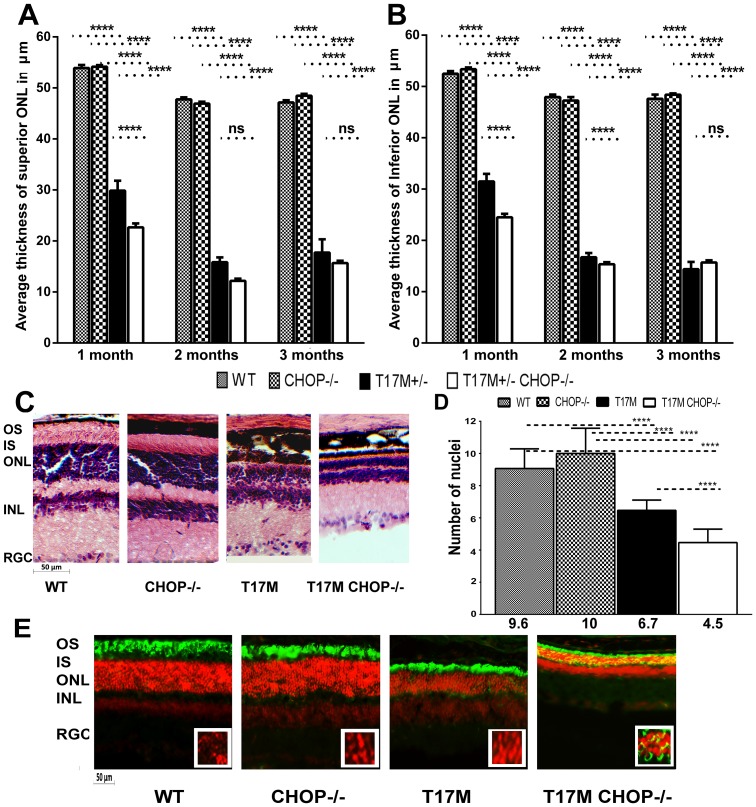
Figure 2. Retinal structure measured by SD-OCT was altered in the T17M RHO CHOP−/− retina.
We analyzed 4 groups of animals (N = 6) by two-way ANOVA and found significant changes in the average thickness of the ONL in the inferior and superior hemispheres in 1,2 and 3-month-old mice. A: In the superior region, the average thickness of the ONL was 53.89 µm ±0.8 in wild-type, vs. 54.2 µm ±0.2 in CHOP−/− mice. A dramatic reduction of 22% in the ONL thickness was observed between the T17M RHO and T17M RHO CHOP−/− retinas (29.88 µm ±0.3 in T17M RHO vs. 23.7 µm ±0.3 in T17M RHO CHOP−/−), which was statistically significant (P<0.001). The differences between wild-type and T17M RHO or T17M RHO CHOP−/− and CHOP−/− and T17M RHO or T17M RHO CHOP−/− were also statistically significant (P<0.0001). No difference in the thickness of the superior ONL was observed when wild-type and CHOP−/− retinas were compared. At 2 months, the ONL thickness in the T17M RHO retina continued to decline and was 15.83 µm ±0.95 in T17M RHO vs. 12.19 µm ±0.43 in T17M RHO CHOP−/−), which was not statistically significant. In wild- type animals, the ONL thickness was 47.8 µm ±0.38 vs 46.92 µm ±0.37 in CHOP−/− mice. The differences between wild-type and T17M RHO or T17M RHO CHOP−/− and CHOP−/− and T17M RHO or T17M RHO CHOP−/− were also statistically significant (P<0.0001). No difference in the thickness of the superior ONL was observed when wild-type and CHOP−/− retinas were compared. At 3 months of age, the ONL thickness in the T17M RHO retina was 17.72 µm ±2.59 in T17M RHO vs. 15.68 µm ±0.43 in T17M RHO CHOP−/−), which was not statistically significant. In wild- type animals, the ONL thickness was 47.15 µm ±0.44 vs 48.47 µm ±0.37 in CHOP−/− mice. The differences between wild-type and T17M RHO or T17M RHO CHOP−/− and CHOP−/− and T17M RHO or T17M RHO CHOP−/− were also statistically significant (P<0.0001). No difference in the thickness of the superior ONL was observed when wild-type and CHOP−/− retinas were compared. B: The average thickness of the inferior ONL was also measured in the 4 groups of mice. We found that the average thickness was 52.5 µm ±0.51 in wild-type; 53.4 µm ±0.3 in CHOP−/− mice vs. 31.5 µm ±0.2 in T17M RHO; and 24.5 µm ±0.4 in T17M RHO CHOP−/−. The differences between wild-type and T17M RHO or T17M RHO CHOP−/− and CHOP−/− and T17M RHO or T17M RHO CHOP−/− were statistically significant (P<0.0001). The difference (24%) between T17M RHO and T17M RHO CHOP−/− was also statistically significant (P<0.001). No difference in the thickness of the inferior ONL was observed when wild-type and CHOP−/− retinas were compared. The average inferior ONL thickness in 2 month-old animals was different in all groups and was 48.0 µm ±0.46 in wild-type; 47.3 µm ±0.75 in CHOP−/− mice vs. 16.7 µm ±0.8 in T17M RHO; and 15.4 µm ±0.4 in T17M RHO CHOP−/−. The differences between wild-type and T17M RHO or T17M RHO CHOP−/− and CHOP−/− and T17M RHO or T17M RHO CHOP−/− were statistically significant (P<0.0001). The difference between T17M RHO and T17M RHO CHOP−/− was also statistically significant (P<0.001). No difference in the thickness of the inferior ONL was observed when wild-type and CHOP−/− retinas were compared. At 3 months of age the difference in the average inferior ONL thickness was not significant between T17M RHO and T17M RHO CHOP−/− mice (14.4 µm ±0.8 in T17M RHO vs 15.6 µm ±0.4 in T17M RHO CHOP−/−) while differences between wild-type (47.6 µm ±0.8) and T17M RHO or T17M RHO CHOP−/− and CHOP−/− (48.4 µm ±0.3) and T17M RHO or T17M RHO CHOP−/− were statistically significant (P<0.0001). No difference in the thickness of the inferior ONL was observed when wild-type and CHOP−/− retinas are compared. C: Histological analyses of wild-type, T17M RHO, T17M RHO CHOP−/− and CHOP−/− retinas: Images of wild-type, T17M RHO, T17M RHO CHOP−/− and CHOP−/− retinas stained with hematoxylin and eosin (H&E). Four animals in each group were used in this experiment. Histology of experimental mouse retinas at 1 month of age showed loss of photoreceptor cell nuclei, shortening of the outer segments, and general disorganization in the T17M RHO retina. Ablation of the CHOP protein in these retinas, however, led to more rapid retinal degeneration that resulted from the shortening of the outer and inner segments and more pronounced general retinal disorganization in the T17M RHO CHOP−/− mice. GCL, retinal ganglion cells; IPL, inner plexiform layer; INL, inner nuclear layer; ONL, outer nuclear layer; IS, inner segments; OS, outer segments. Scale bar indicates 50 µm. D: Photoreceptor cell nuclei in all 4 groups of animals. The number of nuclei was counted by a masked researcher. Two-way Anova with multiple comparison analysis demonstrated differences in all 4 groups of animals (****, P<0.0001) at 1 month of age with the exception of comparison between wild-type and CHOP−/− mice. For example, one-month-old T17M RHO mice had 6.7±0.15 rows whereas the T17M RHO CHOP−/− mice had more severe loss of photoreceptor cells with 4.5±0. 21 rows. These numbers were significantly different from those in the wild type and CHOP−/− animals (9.6±0.31 and 10.0±0.41 respectively). Histological analysis confirmed our OCT data suggesting first, that there is a decline in the number of photoreceptor cells in T17M RHO at 1month and second, the CHOP ablation expedites retinal degeneration in T17M RHO retina. Decline in the number of photoreceptors in these animals was 33% compared to T17M RHO mice. E: Immunohistological analyses of one-month-old wild-type, T17M RHO, T17M RHO CHOP−/− and CHOP−/− retinas. 12 micron cryostat sections of retinas were treated with anti-rhodopsin antibody (in green). The IHC analysis revealed normal localization of rhodopsin in the outer segments of photoreceptor cells in wild type and CHOP−/− retinas. The T17M RHO retinas demonstrated shortening of the OS of photoreceptors (propidium iodide -stained ONL nuclei in red). In the T17M RHO CHOP−/− retina we detected mislocalization of rhodopsin (in yellow) in addition to the shortening of the OS of photoreceptors. Rhodopsin was found in the cytoplasm of photoreceptors around the nuclei (in yellow ONL layer) and this, evidently indicates more severe retinal degeneration compared to T17M RHO mice. RGC, retinal ganglion cells; IPL, inner plexiform layer; INL, inner nuclear layer; ONL, outer nuclear layer; IS, inner segments; OS, outer segments. Scale bar indicates 50 µm.