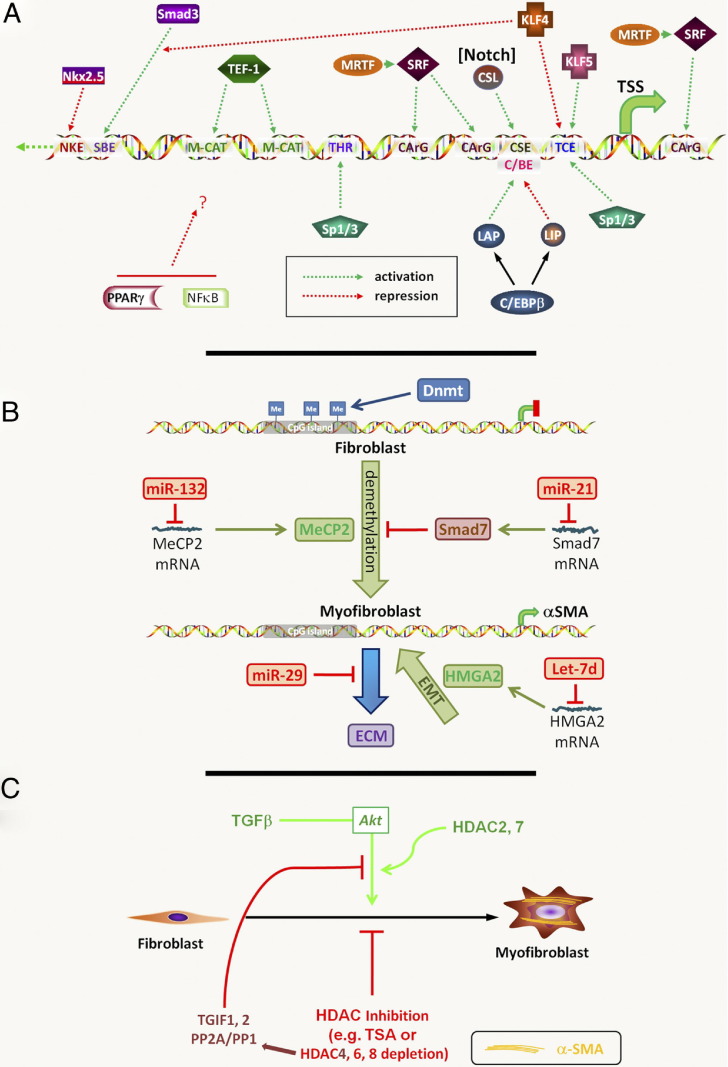
Figure 1.
Myofibroblast epigenetics. A: Transcriptional regulation of the ACTA gene in myofibroblast differentiation. Activating or repressing transcription factors are shown directed at the relative locations of their cognate binding elements in the ACTA gene promoter. The interaction of Kruppellike factor (KLF) 4 with Smad3 results in decreased Smad3 binding to the Smad-binding element (SBE). In contrast, MRTF enhances serum response factor binding to its cognate element to activate differentiation. PPARγ and NF-κB are also shown, but the location/nature of their direct interaction with the promoter is uncertain (indicated with a question mark). The activation of Notch signaling activates its downstream factor CSL. CArG, CC(A/T)6GG; C/EBP, CCAAT enhancer binding protein; CSL, from CBF1/RBP-J in mammals, suppressor of hairless [Su(H)] in drosophila and xenopus, and Lag-1 in Caenorhabditis elegans; IM-CAT, binding element with sequence CATCCT; LAP, liver activator protein; LIP, liver inhibitory protein; NKE, Nkx2.5 binding element; SRF, serum response factor; TEF, transcription enhancer factor; THR, TGF-β1 hypersensitivity region; TSS, transcription start site. B: DNA methylation and miRNAs in myofibroblast differentiation. Methylated CpG islands are shown in the ACTA gene promoter in the fibroblast, which are maintained by DNMT. The differentiated myofibroblast shows reduced methylation with activation of ACTA gene expression. The methylated DNA-binding protein MeCP2 promotes myofibroblast differentiation; however, multiple target genes are regulated by this protein. The indicated miRNA species regulate their target mRNAs with downstream consequences on myofibroblast differentiation, as indicated. Although ACTA is used as a hallmark gene of the myofibroblast, other fibrosis-relevant genes have been regulated by hypermethylation as well (see Myofibroblast DNA Methylation). C: Histone deacetylation and myofibroblast differentiation. The indicated HDAC isoforms modulate differentiation via multiple mechanisms. In addition, inhibition/depletion of HDAC4 suppresses TGFβ activation of Akt via 5′-TG-3′-interacting factor 1,2 (TGIF1,2) and protein phosphatase 2A/1 (PP2A/PP1).