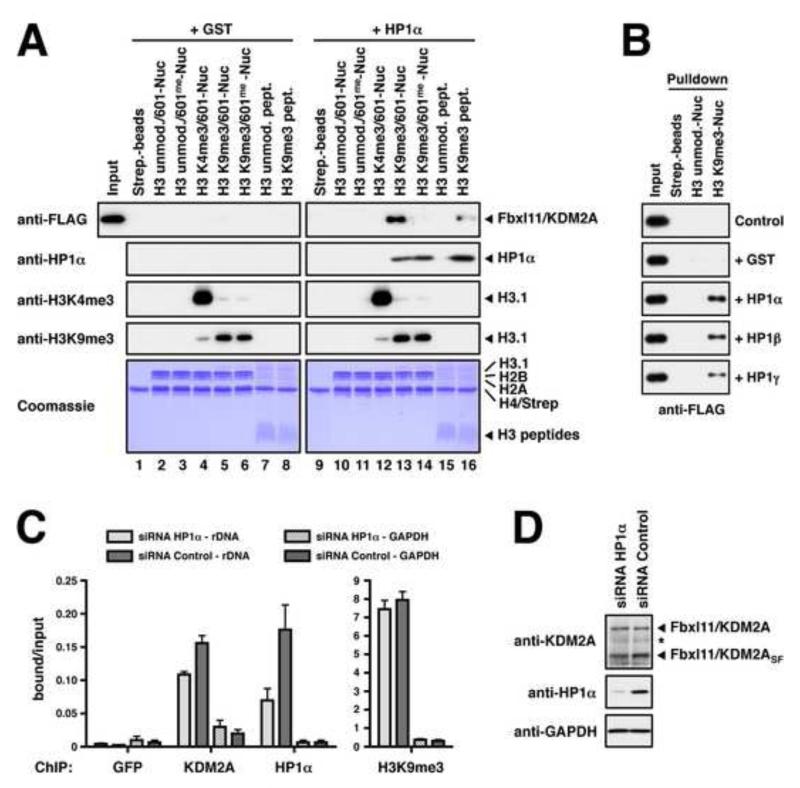
Figure 5. Fbxl11/KDM2A Integrates DNA Methylation and H3K9me3-modification Signals on Nucleosomes.
(A) In vitro binding of KDM2A to modified nucleosomes. Whole cell extracts prepared from transiently transfected 293T cells over-expressing FLAG-tagged KDM2A were incubated with immobilised modified nucleosomes or modified H3-peptides as indicated. Binding reactions were supplemented with recombinant purified HP1α or GST as a control. Binding was detected by immunoblot against the FLAG-tag or HP1α. Equal loading of the nucleosomes and peptides, and modification of histone H3 were verified as in Figure 1D. (B) KDM2A binding to H3K9me3-Nucleosomes is mediated by HP1α, β, and γ. Unmodified or H3K9me3-modified nucleosomes were immobilised on streptavidin beads and incubated with 293T whole cell extracts over-expressing FLAG-tagged KDM2A. Pulldown reactions were supplemented with recombinant purified HP1α, β, or γ or GST as indicated. Binding of KDM2A was detected by immunoblot against the FLAG-tag. (C) Recruitment of KDM2A to the rDNA locus is augmented by HP1α. MCF7 cells were transfected with HP1α-specific siRNAs and analysed for the enrichment of the H13 region of the rDNA locus by ChIP using antibodies against KDM2A, HP1α and histone H3K9me3. Shown are the mean ± SD of the signals normalised to input of three independent experiments. KDM2A shows only little enrichment at the GAPDH locus. (D) Analysis of KDM2A and HP1α expression in siRNA-treated MCF7 cells by immunoblot. GAPDH serves as loading control. See also Figure S4.