Fig. 5.
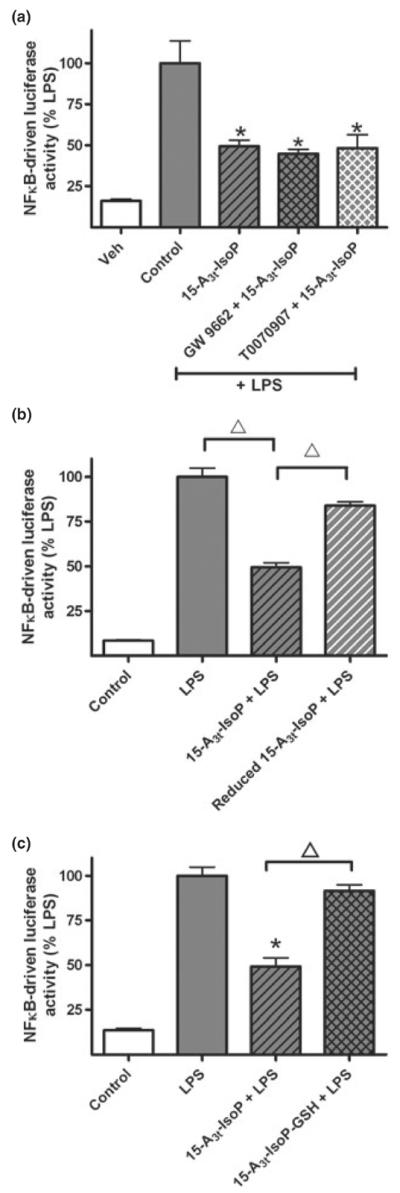
The anti-inflammatory effects of 15-A3t-IsoP are independent of PPARγ activation and are dependent on the α,β-unsaturated carbonyl moiety. (a) NFκB reporter cells were pre-treated with vehicle or one of two PPARγ antagonists (GW9662, 1 μM, or T0070907, 500 nM) for 2 h, exposed to 15-A3t-IsoP (25 μM) for 30 min, and then stimulated with LPS (1 μg/mL) as in previous experiments. Data expressed as % LPS-induced luciferase activity increase. Statistical analysis of was performed using a two-tailed between group anova and demonstrated a significant treatment. Post hoc analysis revealed that LPS only control (gray bar) was significantly different from control (white bar) (a). Additional comparisons revealed 15-A3t-IsoP, 15-A3t-IsoP with GW9662 and 15-A3t-IsoP with T0070907 were significantly different from LPS alone but not from one another as denoted by a * representing a p < 0.01 compared with LPS alone. (b, c) 15-A3t-IsoP was subjected to reduction with NaBH4 for 30 min (b) or incubated with GST and GSH for 2 h (c) and purified by C18 SepPak extraction before being applied to NFκB reporter cells as in previous experiments. C18 SepPak extraction removes residual GST, GSH, and NaBH4. LPS-stimulated NFκB reporter activity was significantly different from control in both panels (b) and (c) and 15-A3t-IsoP's effect on NFκB was significantly diminished when using the reduced form of the compound or the GSH adduct as denoted by bracketed △ representing a p < 0.01 compared with 15-A3t-IsoP + LPS. Data are shown as ± SD from four independent experiments carried out in triplicate.
