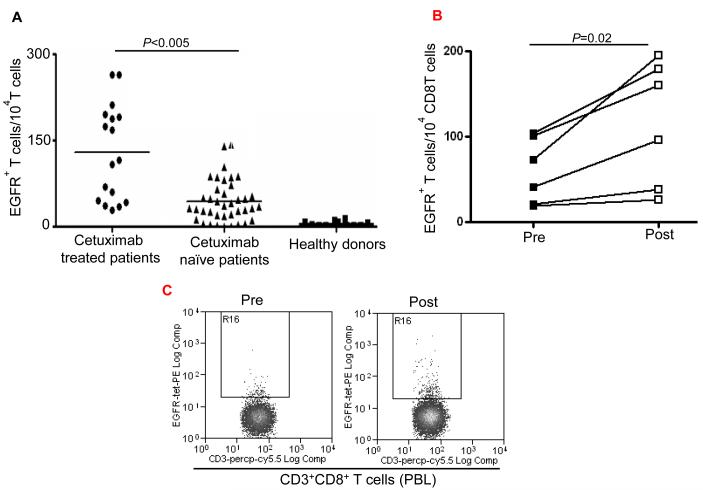
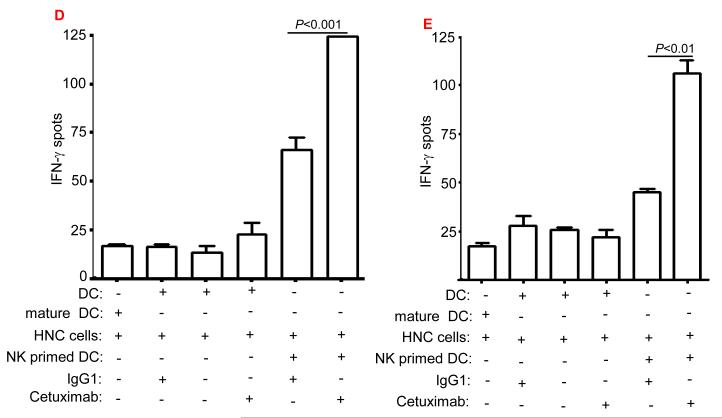
Figure 2.
Enhancement by cetuximab of EGFR853-861-specific CTL in the HNC patients. (A) Higher frequency of EGFR + 853-861 peptide-specific tetramer CD8+T cells in HLA-A2+ cetuximab-treated HNC patients (n=17) compared to HLA-A2+ cetuximab-naïve HNC patients (n=39) was found after EGFR853-861 tetramer staining of CD3+CD8+ T cells by using flow cytometry. The data comparing mean frequencies between cetuximab treated (•) and naïve (▲) patient groups are presented. As a negative control, staining of EGFR853-861 peptide-specific tetramer+ CD3+ CD8+ T cells (from HLA-A2+ healthy donors (n=24) was performed. A two-tailed unpaired t-test was performed for statistical analysis. (B) Expansion of EGFR853-861-specific CTL in single agent cetuximab treated, newly diagnosed HNC patients. Higher frequency of EGFR853-861-specific tetramer+ CD8+T cells in HLA-A2+, single agent cetuximab-treated HNC patients (n=6) after 4 weekly doses using flow cytometry. Mean frequencies were compared from the same patients before ( ■ ) and post ( □ ) cetuximab therapy. (C) A representative dot plot is shown for a single agent cetuximab treated patient (pre and post cetuximab therapy). (D) Enhancement by cetuximab of EGFR cross-presentation to EGFR853- 861-specific CTL by DC that was pre-incubated with cetuximab-activated NK cells. EGFR853-861 peptide-specific CTL were stimulated for 36h at 37°C with DC (from a HLA-A 2.1+ healthy donors) fed with UV-irradiated PCI-15B HNC cells (EGFR+, HLA-2.1−) coated with IgG1 or cetuximab (each at 10μg/ml), with or without autologous NK cells (DC:NK:PCI-15B at 1:1:1 ratio). Cetuximab (IgG1 isotype) binds FcγRIIIa expressed by NK cells avidly. EGFR853-861 peptide-specific CTL activated under the indicated conditions were examined for IFN-γ production by ELISPOT assay. Mature DC generated by 36h incubation with cytokines IL-1β, IL-6, PGE2 and TNF-α were used as a negative control in the assay. Data are representative of three individual experiments. A two-tailed unpaired t-test was performed for statistical analysis. (E) Enhancement by cetuximab of MAGE-3 cross-presentation to MAGE-3271–279-specific CTL by DC that was pre-incubated with cetuximab-activated NK cells. MAGE-3271–279 peptide-specific CTL were stimulated for 36h at 37°C with DC that were prior (from a HLA-A 2.1+ healthy donor) fed with UV-irradiated JHU-029 HNC cells (MAGE-3+, HLA-2.1−) coated with IgG1 or cetuximab (each at 10 μg/ml), with or without autologous NK cells (DC:NK:JHU-029 at 1:1:1 ratio). MAGE-3271–279 peptide-specific CTL activated under the indicated conditions were examined for IFN-γ production by ELISPOT assay. Mature DC generated by 36h incubation with cytokines IL-1β, IL-6, PGE2 and TNF-α were used as a negative control in the assay. We previously showed that an immunogenic EGFR-encoded CTL could be generated by pulsing DC with EGFR853-861 peptide that could stimulate cognate CTL in vitro (14). Total recombinant EGFR protein was not used in this study as a source of TA since this would not mimic FcγRIIIa mediated effects and NK:DC cross talk that cetuximab stimulates. Data are representative of two independent, repeated experiments. A two-tailed unpaired t-test was performed for statistical analysis.