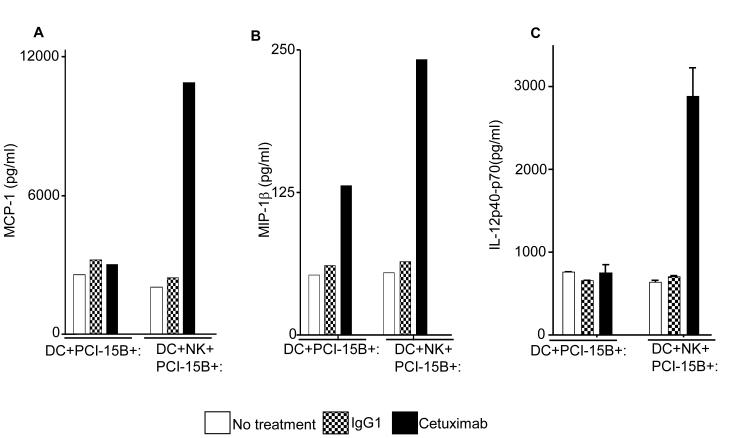
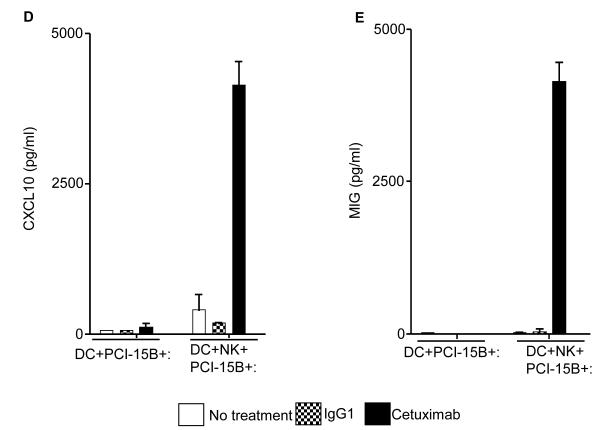
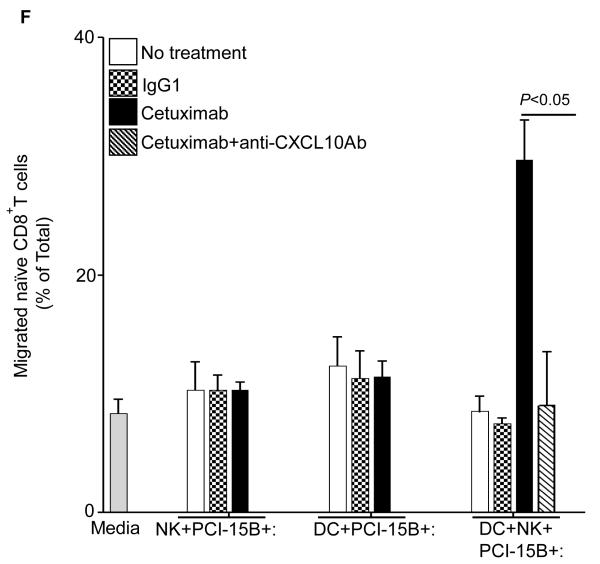
Figure 6.
Enhancement by cetuximab of Th1 polarizing cytokines in the NK:DC:PCI-15B co-culture. (A-C) Luminex™ analysis for the estimation of level of MCP-1, MIP-1β and IL-12 in the supernatant of DC:PCI-15B (1:1 ratio) or DC:NK:PCI-15B (1:1:1 ratio) co-culture with no treatment or with IgG1 or cetuximab (each at 10μg/ml for 48h). Data are representative of two different donors. (D and E) Enhancement by cetuximab of CXC chemokines in the NK-DC co-culture. The levels of CXC chemokines CXCL10 and MIG were determined in the supernatant of from co-culture of DC:PCI-15B (1:1 ratio) or DC:NK:PCI-15B (1:1:1 ratio) incubated with no treatment or with IgG1 or cetuximab (each at 10μg/ml for 48h). Values are mean ± SEM of two independent experiments from separate donors. (F) Enhancement by cetuximab of the migration of CD8+T cells under the influence of CXCL10 in the supernatant of NK:DC:PCI-15B co-cultures. Migration of CD8+ T cells under the influence of migratory factor(s) present in the fresh media or in the supernatant of NK:PCI-15B (at 1:1 ratio) co-culture, or in the supernatant of DC:PCI-15B (1:1 ratio) co-culture, or in the supernatant of DC:NK:PCI-15B co-culture (1:1:1 ratio), with no treatment or with IgG1 or cetuximab (each at 10μg/ml for 48h) was quantified. After 4h of incubation of CD8+ T cells with co-culture supernatants in the upper chamber of transwell plate, cells were collected from lower chamber and counted. Each condition was plated in triplicate. In parallel to the cetuximab condition anti-CXCL10 Ab (10 μg/ml) was used for the blocking experiments. Data shown are from a representative donor performed in triplicate. Two-tailed unpaired t-test was performed for statistical analysis.