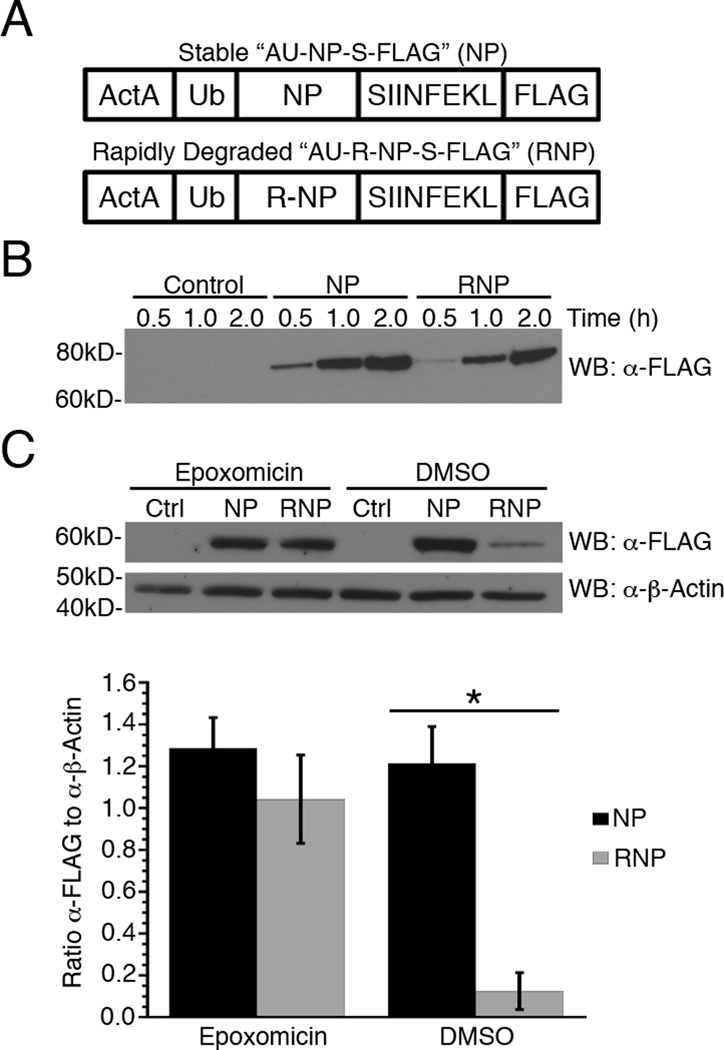
Figure 1. Generation of stable and rapidly degraded forms of a recombinant protein expressed by Listeria.
(A) Schematic representation of recombinant proteins expressed from Listeria. The NP construct is comprised of the first 100 amino acids of ActA, followed by full-length ubiquitin (Ub) and influenza nucleoprotein (NP), the minimal Kb-binding peptide SIINFEKL (S) and a 3×FLAG® tag (FLAG). The RNP construct is identical to NP except the amino-terminal methionine residue of NP has been replaced with arginine. (B) NP and RNP synthesis and secretion from recombinant Listeria in eukaryotic cell culture medium were determined by quantitative Western blot analysis using a monoclonal anti-FLAG antibody. The control is eukaryotic cell culture medium inoculated with Listeria that does not express a FLAG-tagged protein. (C) Secretion of NP and RNP into Listeria infected BMA3 cells was determined in the presence or absence of 5 μM epoxomicin by quantitative Western blot analysis as in (B) above. FLAG concentrations were normalized to β-actin for three independent experiments and data are shown as mean ± SEM, *,p<0.05.