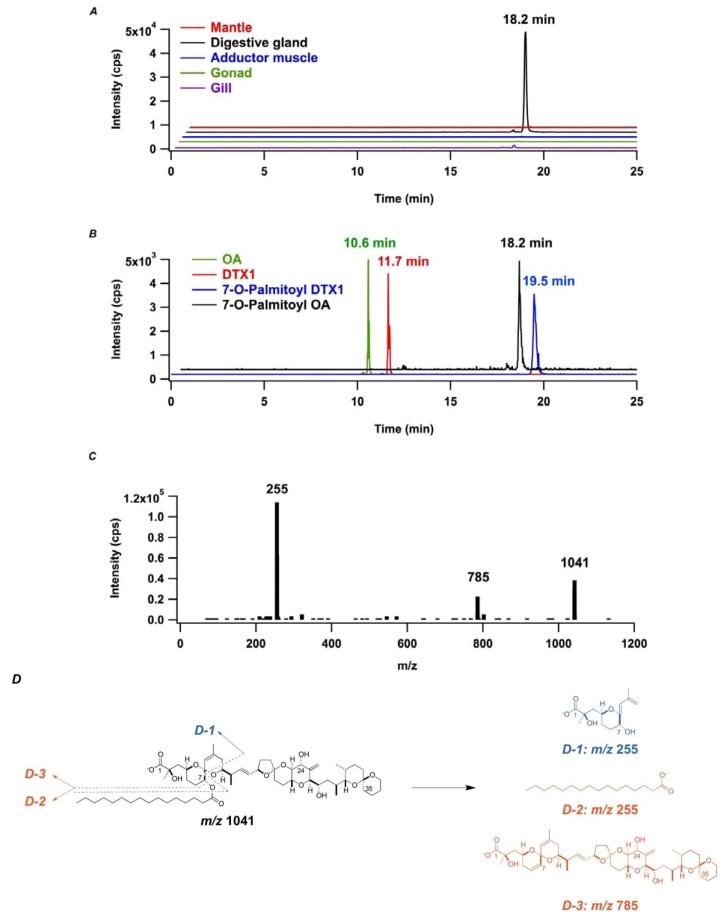
Figure 3.
Identification of 7-O-palmitoyl OA in in vitro transformation reaction mixture. (A) In vitro transformation reactions were performed in the presence of microsomal fractions prepared from different tissues of Mizuhopecten yessoensis. From the front side to the backside, the microsomal fraction was prepared from the gill (purple), gonad (green), adductor muscle (blue), digestive gland (black) and mantle (red) and was subjected to in vitro acylation reactions. The production of 7-O-palmitoyl OA was confirmed by LC-MS/MS analysis on multiple reaction monitoring (MRM) negative ion mode with m/z 1041 > 255 as precursor ion/product ion; (B) Mass chromatograms for m/z 1041 > 255 from sample (black) and three mass chromatograms for m/z 803 > 255 (green, OA), m/z 817 > 255 (red, DTX1) and m/z 1055 > 255 (blue, 7-O-Palmitoyl DTX1) from diarrhetic shellfish poisoning (DSP) standard were obtained from LC-MS/MS on MRM negative ion mode. The latter three chromatograms were overlapped and shown in the front side; (C) The MS/MS spectrum for 7-O-palmitoyl OA. The peak at 18.2 min in the mass chromatogram in (A) was analyzed with LC-MS/MS on enhanced product ion (EPI) scan mode. (D) The estimated structures for the three product ions m/z 1041, m/z 785 (D-3) and m/z 255 (D-1, D-2) observed in the MS/MS spectra in (C).