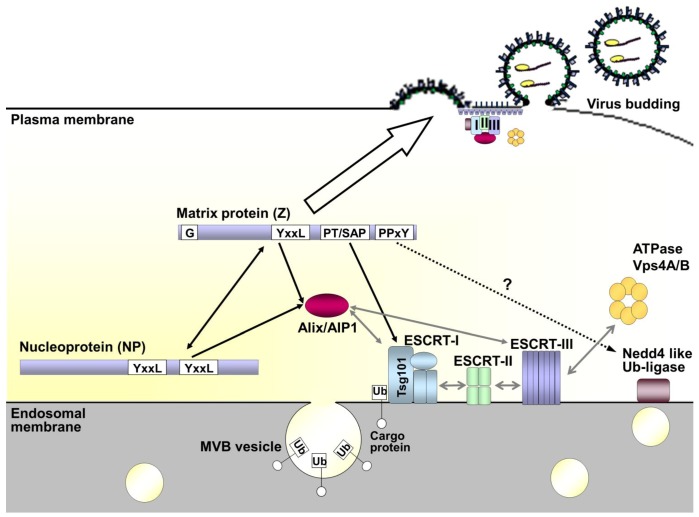
Figure 6.
Model of arenavirus budding and the role of the ESCRT pathway. Under normal cellular conditions the ESCRT pathway is involved in vesicular trafficking of cargo proteins through multivesicular bodies (MVBs). This is facilitated by ubiquitination of the cargo protein, which mediates recognition by and sequential recruitment of the ESCRT-I, -II and -III complexes. Alix/AIP1 can also act to connect the ESCRT-I and -III complexes directly. Once recruited the ESCRT-III component is responsible for membrane scission, thereby releasing the newly formed vesicle. Finally, Vps4A/B mediates disassembly and recycling of the entire complex. During viral infection interaction of the matrix protein Z with components of the ESCRT pathway takes place through late domain motifs. The sequences are present in Z and vary between arenaviruses, but include some combination of PPxY, PT/SAP and/or YxxL sequences. These late domain motifs allow interaction with Nedd 4-like ubiquitin ligases, Tsg101 (a component of ESCRT-I) and Alix/AIP1, respectively. As a consequence of its interaction with these various ESCRT components and ESCRT-associated proteins, Z serves to redirect these components to the sites of budding at the cell membrane. This ability of Z to interact with the cell membrane is critically dependent on myristoylation of the glycine residue (G) at amino acid position 2 in the protein sequence. For some arenaviruses additional interaction of late-domain motifs in NP with Alix/AIP1 may further support budding, while also serving to recruit nucleocapsids into the forming virus particle through a direct Z-NP interaction.