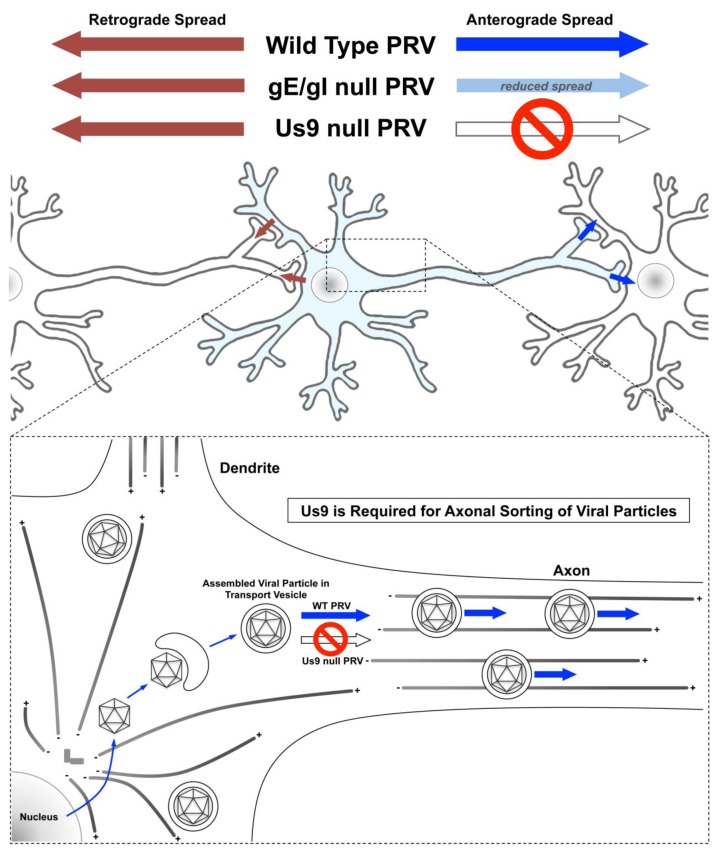
Figure 3.
PRV Us9 and gE/gI mediate anterograde spread of infection in neurons. PRV Us9 is essential for anterograde trans-neuronal spread in vivo and in vitro. In the absence of Us9, viral particles are assembled in the cell body, but are not sorted into axons. This neuron-specific phenotype suggests that Us9 functions by recruiting a molecular motor protein, either directly or indirectly, that facilitates axonal sorting of viral particles into axons. In contrast, gE/gI null mutants are defective for anterograde trans-neuronal spread in vivo, but are able to spread with reduced capacity in vitro. gE/gI null mutants display a small plaque phenotype in non-neuronal cell types.