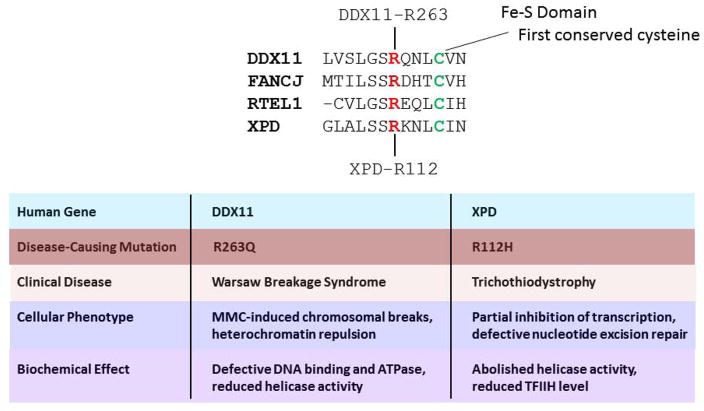
Figure 3. Pathogenic mutation of homologous residue in Fe-S domain of DDX11 and XPD.
Amino acid alignment showing start of Fe-S cluster in human DDX11, XPD, FANCJ, and RTEL1 helicase proteins. Mutation of conserved arginine (R263 in DDX11, and R112 in XPD (red)), residing four residues upstream of highly conserved cysteine (green), is responsible for WABS and TTD, respectively. Distinguishing cellular phenotypes and biochemical effects of the DDX11 and XPD mutations linked to WABS and TTD, respectively, are noted. See references [73–75] and text for details.