Figure 7. Uba6-Use1 and Uba1-UbcH7 pathways ubiquitylate Ube3ain vitroand spatially control Ube3a abundance in MEFs.
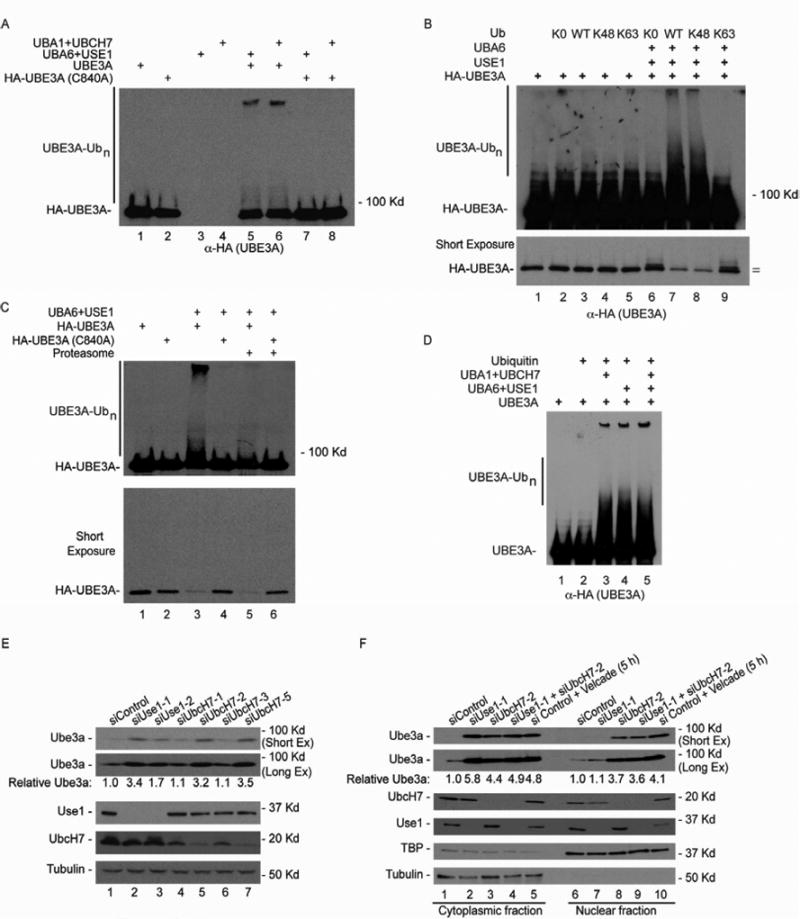
(A) HA-UBE3A (purified from 293T cells) with or without mutation of its active site cysteine residue (C840A) was incubated with ATP, ubiquitin, and UBA6-USE1 or UBA1-UBCH7.
(B) USE1 promotes K48-linked ubiquitylation of UBE3A. The “=” indicates the positions of HA-UBE3A and its mono-ubiquitin conjugate migrating more slowly.
(C) Ubiquitylated UBE3A is degraded by the 26S proteasome. The indicated reaction mixtures were treated with of proteasomes (360 nM) in which associated ubiquitin specific proteases were inhibited with ubiquitin-vinyl-sulfone.
(D) Patterns of UBE3A ubiquitylation in vitro are indistinguishable with UBA6-USE1, UBA1-UBCH7, or both pathways together.
(E) Use1 and UbcH7 independently function to control Ube3a abundance in MEFs. The indicated siRNAs were transfected into MEFs and the levels of the indicated proteins accessed after 72 hours using western blotting. Tubulin as used as a loading control.
(F) Spatially distinct control of Ube3a in MEFs via Uba6-Use1 and Uba1-UbcH7 pathways. As in (E), except that cytoplasmic and nuclear fractions were isolated and analyzed separately by immunoblotting.
