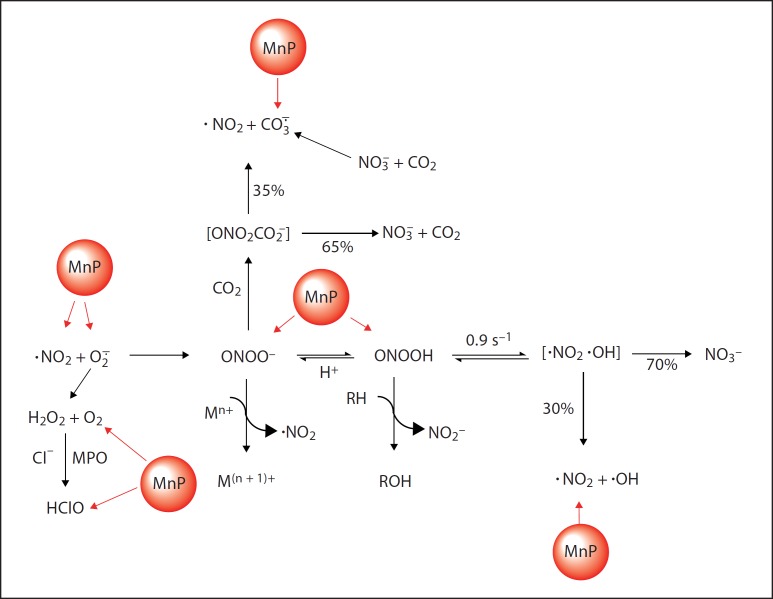
Fig. 6.
The reactivity of MnPs towards reactive species is related to the electron deficiency of the metal site characterized by E1/2 (see fig. 3). The most potent Mn porphyrins have a very electron-deficient metal site and thus favor binding of electron-donating anionic species such as O2–·, ONOO–, CO3–·, ClO–, etc. Further due to the same fact they favor accepting electrons and being readily reduced with cellular reductants; in a subsequent step (while being oxidized from MnIIP to MnIIIP) MnIIP can reduce the reactive species. The cationic character allows them to approach the anionic deprotonated cysteines of signaling proteins and oxidize those while undergoing reduction. When oxidized to O = MnIVP4+ (with ONOO–, H2O2, ClO– or CO3–·), in a subsequent step they readily oxidize glutathione, ascorbate or uric acid and undergo reduction to MnIIIP whereby closing the catalytic cycle. Adapted from Batinic-Haberle et al. [7].