Fig. 8.
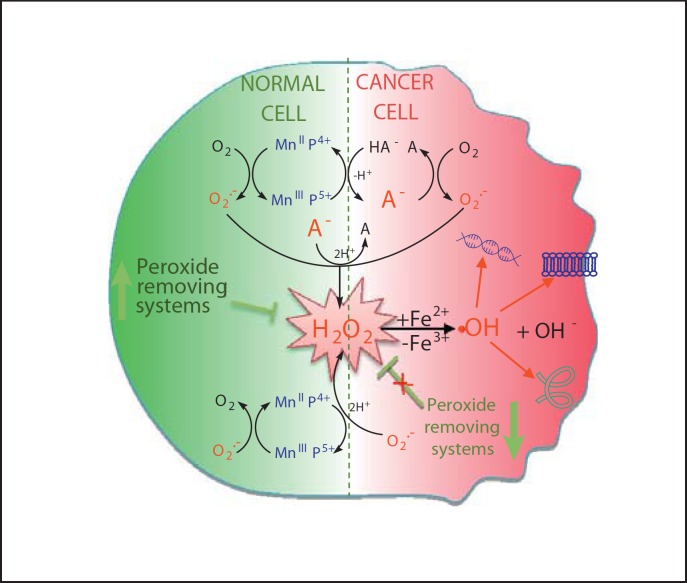
Mn porphyrin redox cycling with ascorbate will cause differential effects in cancer and normal cells. Due to the high cellular levels of ascorbate, it is likely that cationic Mn(III) N-alkylpyridylporphyrins will be reduced with ascorbate to MnIIP within the cell, which will then act as a reductant, either reacting with O2–· or ONOO– or ClO– or H2O2 closing the catalytic cycle. Due to the abundance of oxygen relative to the levels of other species, MnIIP may prefer reducing O2 to O2–· which will eventually dismute to H2O2. The normal cell has the abundance of peroxide-removing enzymes, thus the contribution of Fenton chemistry leading to a deleterious –OH radical may be negligible [203,204,205,206,207,208,209,210,211]. Cancer cells are frequently deprived of H2O2-removing enzymes, thus excessive amounts of peroxide will be formed. In such a situation, coupling with ascorbate involves O2 or O2–· (catalytic in nature) and may produce pro-oxidative effects, and favor cancer cell death as opposed to normal, nontransformed cells [9,27,148].