Structured Abstract
Objective
To determine if deficiency of nitric oxide bioactivity contributes to the physiologic instability that occurs following brain death and, if so, to also determine in this setting whether administration of a renitrosylating agent could improve systemic physiologic status.
Summary Background Data
Organ function following brain death is negatively impacted by reduced perfusion and increased inflammation; the magnitude of these responses can impact post-graft function. Perfusion and inflammation are normally regulated by protein S-nitrosylation but systemic assessments of nitric oxide bioactivity following brain death have not been performed.
Methods
Brain death was induced in instrumented swine by inflation of a balloon catheter placed under the cranium. The subjects were then serially assigned to receive either standard supportive care or care augmented by 20 ppm of the nitrosylating agent ethyl nitrite blended into the ventilation circuit.
Results
Circulating nitric oxide bioactivity (in the form of S-nitrosohemoglobin) was markedly diminished 10 h after induction of brain death—a decline that was obviated by administration of ethyl nitrite. Maintenance of S-nitrosohemoglobin was associated with improvements in tissue blood flow and oxygenation, reductions in markers of immune activation and cellular injury, and with preservation of organ function.
Conclusions
In humans the parameters monitored in this study are predictive of post-graft function. As such, maintenance of endocrine nitric oxide bioactivity after brain death may provide a novel means to improve the quality of organs available for donation.
Introduction
Transplantation is an accepted intervention to correct innate organ failure but a successful outcome is highly dependent upon the physiologic status of the donor. On average, more than 75% of transplanted organs are recovered from deceased individuals, the majority of which are classified as brain dead heart beating donors (Organ Procurement and Transplantation Network; http://optn.transplant.hrsa.gov/). Five year survival rates for recipients of these organs range from 80% for kidneys to just over 50% for lungs; where comparisons can be made (kidney, liver) survival rates are significantly higher for those patients who received their new organ from a living donor1, 2. Stressors placed upon organs, particularly brain death, are believed to contribute to these survival rate differences. Stressors are also significant contributors to the poor procurement rate of suitable organs from consented donors (<50%) due primarily to in situ organ failure3.
The period during and following brain death is characterized by physiologic instability, the result first of intense sympathetic stimulation and significant release of catecholamines (the Cushing Response) with transient vasoconstriction, hypertension, and tachycardia, followed by the complete loss of sympathetic activity leading to hypotension and a profound reduction in systemic vascular resistance4. Hemodynamic homeostasis is regulated in part by nitric oxide (NO)5. A disruption of endocrine NO bioactivity may contribute to the tissue ischemia and organ damage that has previously been ascribed to loss of autonomic function. Post-brain death organ function can be further impaired by an inflammatory state6, and this too may reflect a deficiency of NO7.
Once brain death is confirmed, care of the patient switches from restorative to supportive as organ donation status is assessed8. Supportive care aims to maintain organ perfusion and, to some extent, the acidosis and systemic effects of brain death can be controlled by altering ventilation rates and/or administration of vasoactive agents. However, such interventions may have minimal ability to preserve end-organ oxygen delivery, which is primarily a function of local tissue perfusion. Local blood flow is “auto-regulated” at least in part through NO bioactivity derived from red blood cells9, 10. Hemoglobin (Hb) plays a central role in this auto-regulation by coupling the binding and release of oxygen with that of NO11. Hypoxic vasodilation by red blood cells is linked to Hb desaturation, which effects release of NO from thiols of Hb (S-nitrosoHb; SNO-Hb), thereby providing a regulated mechanism for matching blood flow (oxygen delivery) with local metabolic demand5.
Decreased levels and/or impaired processing of SNO-Hb have been observed in diseases characterized by tissue hypoxemia12–18; where examined, red blood cells from these disparate patient populations exhibited impaired vasodilatory capacity. Such data suggest that red blood cell-derived NO bioactivity may play an important role in the respiratory cycle and that impairment of this activity might contribute to the pathophysiology of ischemic conditions. Based on these findings, we reasoned that a similar disruption of this endocrine function of red blood cells might occur following cessation of central nervous system activity, thus contributing to systemic hemodynamic instability. We further reasoned that in this setting an intervention directed towards increasing NO bioactivity could be beneficial.
Methods
This study was approved by the Duke University IACUC and all procedures complied with The Guide for the Care and Use of Laboratory Animals and the Animal Welfare Act. Experiments were conducted on young adult swine with the experimental treatment cohort receiving 20 ppm of the S-nitrosylating agent ethyl nitrite (ENO; blended in nitrogen by Custom Gas Solutions of Durham, NC).
Surgical Instrumentation
Animals were sedated with acepromazine (0.8 mg/kg) and ketamine (20 mg/kg; both im) then anesthesia was induced with thiopental (10 mg/kg, iv). After endotracheal intubation, anesthesia was maintained with isoflurane (1.5 to 2.0%). Ventilatory settings were adjusted to maintain end-tidal CO2 below 35 mm Hg. Isoflurane and end-tidal CO2 concentrations were continually measured by an airway gas monitor (Datex Instrumentation Corporation, Helsinki Finland). Body temperature was maintained at 38 (±0.5) °C with a Bair-Hugger warming system (Arizant Healthcare Incorporated, Eden Prairie, MN). EKG leads were placed, vascular access was obtained at various sites (right internal jugular for the central line, femoral artery and vein for the peripheral lines), and bladder access was secured through a peritoneal incision. Hemodynamic parameters (cardiac output, arterial pressures, etc) were monitored using a Vigilance system from Edwards Lifesciences (Irvine, CA). After revealing the scalp, a small hole was bored for placement of an intra-cranial pressure (ICP) probe (Aesculap Inc. USA, Center Valley, PA) and a forehead sensor was placed to monitor brain electrical activity (Aspect Medical Systems, Inc. Norwood, MA). A second trepanation was conducted for insertion of a balloon catheter.
Brain Death
Brain death was induced by inflation of the balloon catheter over a period of 10–15 min until ICP exceeded systolic blood pressure. This state of elevated ICP was maintained throughout the study to keep cerebral perfusion pressure at 0 mm Hg. Loss of brain electrical activity was confirmed and then the vaporizer was turned off. When expired isoflurane concentration reached 0, a brain death assessment was conducted (pupils fixed and non-reactive to light, no lash or corneal touch response, absent oculo-cephalic reflex, absent vestibulo-ocular reflex following auricular washing with cold saline, no gag reflex, and no cough with tracheal suction). A ventilatory drive test was not conducted because we determined during model development that this procedure induced cardiac instability. ENO treatment was begun after confirmation of brain death and continued for 10 h. Cardiac instability was treated with fluids and pharmacologic therapy19; hormone replacement was not initiated. Ventricular fibrillation refractory to electro-cardioversion was cause for early study termination.
Physiologic Analyses
Tissue oxygenation and tissue Hb levels (the latter serving as a surrogate for tissue blood flow) were measured in the buccal mucosa using a spectrophotometric monitoring system (T-Stat; Spectros Corporation, Portola Valley CA)20. Arterial and venous blood gas parameters were measured at 60 min intervals during the study using a Gem Premier 3000 (Instrumentation Laboratory, Lexington, MA) Additional venous blood samples were collected before and then again 10 h after the determination of brain death for clinical chemistry analyses (Antech Diagnostic; Cary, NC) and for determination of red blood cell SNO-Hb levels, the latter using mercury-coupled photolysis-chemiluminescence21, 22.
Glomerular filtration rate (GFR) was estimated from the serum chemistry data using the following formula: 170 × [creatinine]−0.999 × [blood urea nitrogen]−0.170 × [albumin]+0.318, modified from Levey et al23. Inulin clearance was another methods used to measure kidney function: 60 mg/kg of inulin (Alfa Aesar, Ward Hill, MA) in 250 ml of normal saline was rapidly infused (iv), urine was collected for 1 h, and at the end of this collection period a venous blood sample was obtained; inulin clearance assessments were also conducted before and then started 9 h after brain death to account for the 60 min interval between inulin infusion and blood/urine collection. Blood and urine inulin levels were then quantitated using an established chromatographic method24, 25.
Statistical Analyses
Data are presented as means ± standard deviations (SD) except for changes in oxygen content, which are presented as medians ± first and third quartile deviations. For finite parameters, testing involved paired t-tests to determine differences before and after brain death; where significant changes were identified analysis of variance (ANOVA) was used to test if the magnitude of the changes were different between groups.
To determine change in oxygen utilization, arterial and venous blood oxygen contents were calculated using the standard clinical formula ([blood oxygen saturation × Hb × 1.34] + [0.003 × blood oxygen partial pressure]) from which the difference was determined by subtracting the venous value from the arterial value. Subsequent changes in the A/V difference were determined by subtraction: A/Vexperiment – A/Vbaseline. Positive values indicate an decrease in venous blood oxygen content and thus an increase in oxygen utilization since arterial oxygen content was essentially constant; negative values reflect an increase in venous blood oxygen content and thus an reduction in oxygen utilization.
For continual parameters, median values were calculated at 60-min intervals and averaged to obtain group data for presentation. Area under the curve values for percent change from baseline of tissue oxygenation and tissue-Hb, and of the A/V differences were calculated and then tested for treatment effects using ANOVA. Tukey’s test was used to identify group differences when the F statistic was significant. Linear regression, where noted, was conducted to test for temporal relationships. Comparisons were made using the Prism Graphpad software package (Graphpad, La Jolla, CA); p values of < 0.05 were considered significant.
Results
General Physiologic Status
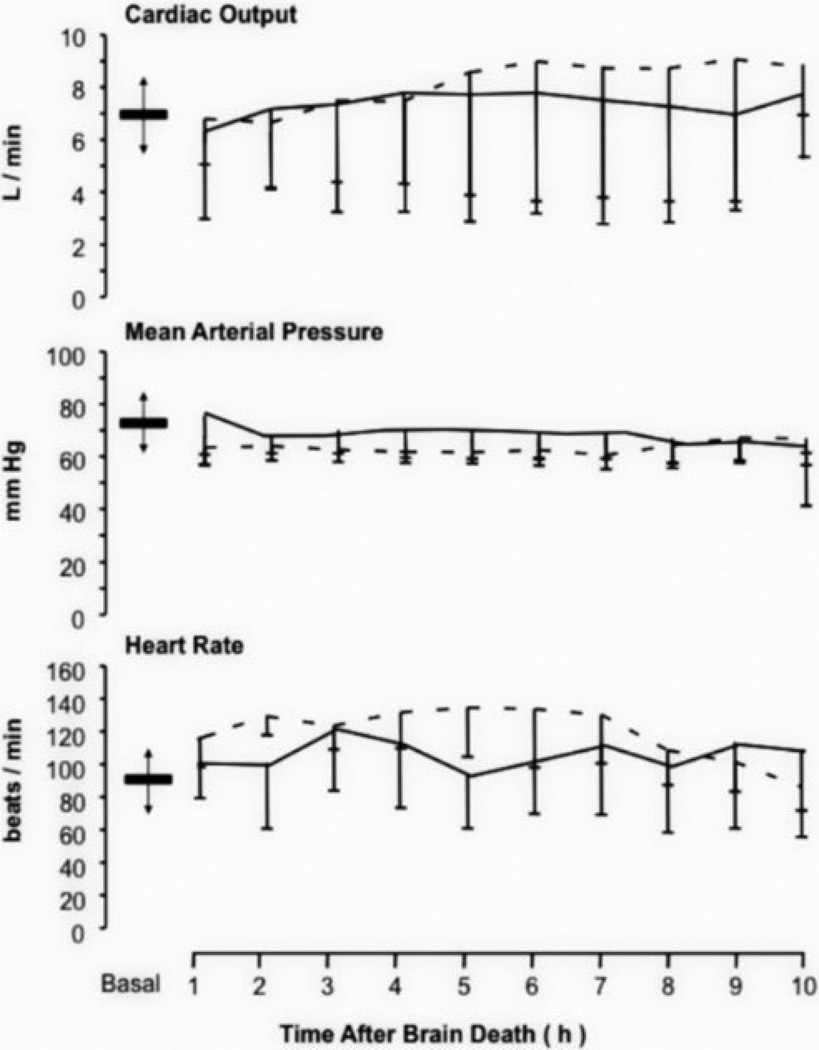
A total of 39 adult swine were utilized for this study: 16 were employed for model development and initial ENO dose titration assessments; 3 developed intractable ventricular fibrillations and cessation of cardiac activity after induction of brain death but before the end of the study (2 in the control group and 1 in the ENO group); and 20 completed the study (n=10 per group). Data on the cardiovascular status for these latter swine are presented in Figure 1. Parameters for all animals that completed the study met the United Network for Organ Sharing guidelines, specifically cardiac output was > 3.8 l/min and mean arterial pressure was > 60 mm Hg. Ventilation was actively regulated to keep end-tidal CO2 below 35 mm Hg and the PaO2/FiO2 ratio significantly higher than 300 mm Hg (data depicted in the Supplemental Digital Content Figure 1).
Figure 1.
Cardiovascular status. Mean (± SD) time courses for cardiac output, mean arterial pressure, and heart rate after the determination of brain death for the untreated (solid line) and ethyl nitrite (ENO) exposed (small dashes) cohorts. Within each graph, baseline mean (± SD) values are depicted by the bar and arrows. These parameters met the United Network Organ Sharing guidelines for donor maintenance with cardiac output > 3.8 l/min and mean arterial pressure > 60 mm Hg. Assessing for cardiovascular treatment differences was not a component of this study.
SNO-Hb, Brain Death, and Tissue Oxygenation
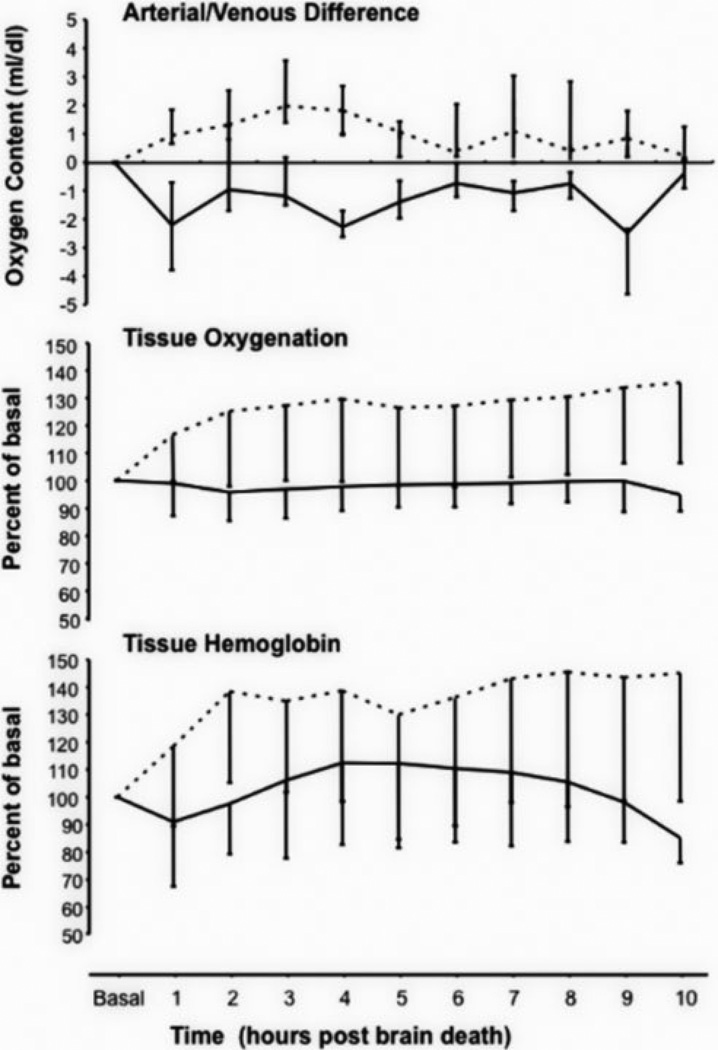
SNO-Hb levels were measured in venous blood samples obtained after securing vascular access and then again 10 h after the determination of brain death (Table 1). In the control cohort, SNO-Hb concentrations declined from 0.52 ± 0.68 to 0.21 ± 0.22 moles of SNO per moles of Hb tetramer × 10−3 (p=0.04 paired t-test; n=8 with two samples lost during processing) whereas there was minimal decline in SNO-Hb in the 20 ppm cohort (0.38 ± 0.64 to 0.35 ± 0.15 SNO per Hb tetramer × 10−3; p>0.05, n=9 with one sample lost during processing). The decline in SNO-Hb in the control group was associated with alterations in oxygen utilization and local blood flow (Figure 2) as demonstrated by the changes from baseline in A/V oxygen content differences and buccal skeletal muscle bed tissue oxygenation and tissue-Hb values. In the control group, the changes in A/V difference following brain death were negative, indicative of a rise in venous oxygen content. This was accompanied by and correlated with (p<0.001) declines in both tissue oxygenation and tissue blood flow (i.e. a reduction in tissue Hb). ENO treatment reversed these effects: the A/V oxygen content difference was greater after brain death (i.e. venous blood oxygen content declined) and tissue oxygenation and tissue blood flow increased. Similar to the control group, the changes in A/V oxygen content correlated with tissue oxygenation and tissue blood flow (p<0.001). Area under the curve comparisons determined that the three parameters were all significantly different between the control and 20 ppm ENO groups (p=0.028, p=0.0037, and p=0.021 for A/V content, tissue oxygenation, and tissue Hb, respectively).
Table 1. Metabolic Parameters.
Data on S-nitroso hemoglobin (moles of SNO per moles of Hb tetramere × 10−3) along with various serum enzymes and metabolic markers before and 10 h after determination of brain death for the two experimental groups.
| Parameter (units) | Control | 20 ppm ENO | ||
|---|---|---|---|---|
| before | after | before | after | |
| SNO-Hb (Hbx10−3) | 0.52 ± 0.68 | 0.21 ± 0.22* | 0.28 ± 0.64 | 0.35 ± 0.15 |
| Glucose (mg/dl) | 95 ± 32 | 120 ± 35* | 91 ± 24 | 113± 32 |
| Total Protein (g/dl) | 5.9 ± 0.6 | 5.1 ± 0.6* | 5.5 ± 0.4 | 4.6 ± 0.4* |
| Lipase (U/l) | 15 ± 28 | 45 ± 30* | 3.8 ± 3.6 | 41 ± 48* |
| Amylase (U/l) | 2755 ± 1531 | 2842 ± 1234 | 2084 ± 687 | 2110 ± 984 |
| Alk Phosphatase (U/l) | 128 ± 29 | 127 ± 29 | 137 ± 35 | 149 ± 48 |
| Creatinine (mg/dl) | 1.7 ± 0.3 | 2.1 ± 0.5* | 1.9 ± 0.3 | 1.9 ± 0.4 |
| ALT (U/l) | 27 ± 6 | 48 ± 33* | 30 ± 8 | 34 ± 8 |
| AST (U/l) | 37 ± 12 | 248 ± 175*† | 37 ± 11 | 147 ± 64* |
| CPK (U/l) | 2963 ± 1368 | 32418 ± 18770*† | 3444 ± 1377 | 16422 ± 6730* |
| Hemoglobin (g/dl) | 10.6 ± 1.5 | 11.4 ± 1.8* | 10.5 ± 0.9 | 11.2 ± 1.4 |
| Hematocrit | 0.33 ± 0.05 | 0.34 ± 0.05 | 0.32 ± 0.03 | 0.34 ± 0.04 |
| WBC (per mm3) | 12.5 ± 3.4 | 15.7 ± 3.4* | 13.4 ± 2.7 | 12.6 ± 3.9 |
| Neutrophils (cell count) | 5940 ± 2621 | 8304 ± 2215* | 6558 ± 1404 | 5453 ± 2684 |
| Monocytes (cell count) | 245 ± 152 | 939 ± 620* | 285 ± 151 | 208 ± 231 |
Values in the “after” columns that are followed by an asterisk (*) indicate a significant difference from the pre brain death values (p<0.05).
Values in the “after” column of the control group also followed by a cross (†) indicate that the magnitude of the increase was significantly larger than the change observed in the ENO treatment group (again, p<0.05).
Abbreviations: ENO, ethyl nitrite; SNO-Hb,; Alk, alkaline; ALT, alanine aminotransferase; AST, aspartate aminotransferase; CPK, creatine phosphokinase.
Figure 2.
Oxygen utilization and tissue oxygenation. Median (± QD) changes in arterial/venous (A/V) oxygen content differences along with mean (± SD) tissue oxygenation and tissue hemoglobin (a measure of blood flow) percent changes from baseline after the determination of brain death for the untreated (solid line) and ethyl nitrite (ENO) exposed (small dashes) cohorts. A/V values were determined by the formula A/Vexperimental – A/Vbaseline. Negative values, as seen in the control group, reflect an increase in venous blood oxygen content following brain death and thus a reduction in oxygen utilization since arterial oxygen content was constant. The positive values, as seen with the ENO treatment, indicate a decrease in venous blood oxygen content and thus an increase in oxygen utilization. Tissue oxygenation and tissue hemoglobin were both significantly increased by 20 ppm ENO compared to the control values.
Metabolic Parameters
Values for various serum enzymes and metabolic markers before and 10 h after confirmation of brain death are presented in Table 1. (Additional serum chemistry results can be found in the Supplemental Digital Content Table 1.) Changes were most notable in the control group. Serum glucose levels increased, presumably reflecting an increase in glycogenolysis26. Total protein decreased, which along with the changes in serum electrolytes and increase in urine volume is indicative of induction of diabetes insipidus27. Lipase (but not amylase or alkaline phosphatase) increased in both groups. Markers of liver (alanine aminotranferease, ALT; aspartate aminotransferase, AST), kidney (creatinine), and tissue (creatine phosphokinase; CPK) injury were all significantly elevated in the control animals. While AST and CPK also increased in the ENO group, the magnitudes of these increases were significantly smaller. Modest elevations in hematocrit, and hemoglobin occurred in both groups. White blood cell (WBC) count only rose in the control group, which represented statistically significant increases in circulating neutrophils and monocytes.
Kidney Function
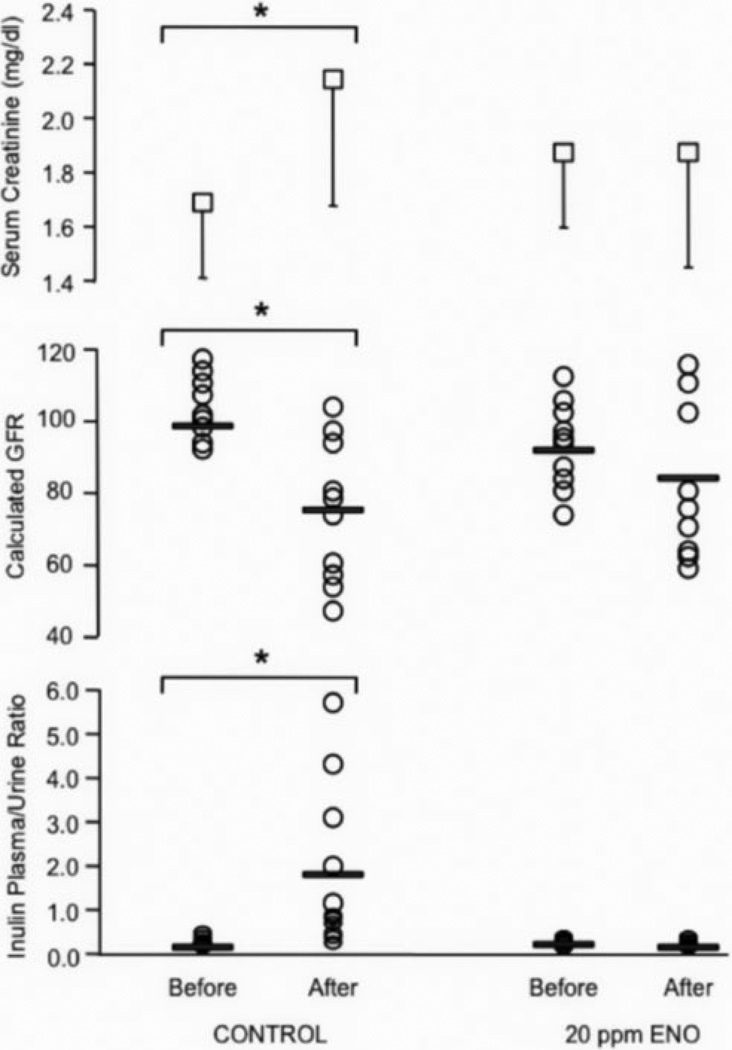
GFR and inulin clearance were assessed before and after brain death with clearance calculated as the plasma to urine inulin concentration ratio. These parameters along with mean serum creatinine levels are presented in Figure 3. In the control group, the mean inulin/plasma ratio increased significantly 10 h after brain death (from 0.15 to 1.89; p=0.011 paired t-test). This was accompanied by a significant decline in GFR (from 102 ± 13 to 77 ± 18 arbitrary units; p=0.0009) and a significant increase in serum creatinine (p=0.02). In contrast, neither the before/after inulin ratios (0.21 versus 0.17; p=0.49) nor GFR (91 ± 15 versus 84 ± 22; p=0.10) were different in the 20 ppm ENO group nor was there a change in the treated subjects’ serum creatinine (p=0.90).
Figure 3.
Kidney function and serum creatinine. Mean (± SD) serum creatinine (top panel), calculated individual glomerular filtration rates (GFR; middle panel), and individual inulin plasma/urine ratios (bottom panel) before and 10 h after determination of brain death. The group means for GFR and inulin ratios are demarcated with bars. Serum creatinine and the plasma/urine inulin ratios significantly increased and GFR significantly decreased in the untreated control cohort after brain death (p < 0.05; brackets with asterisks). In the 20 ppm ethyl nitrite (ENO) treatment group, the before and after brain death values for all three parameters were not statistically different.
Discussion
Altered levels of SNOs and impaired vasodilatory activity of red blood cells have been observed in several chronic disease states characterized by aberrant tissue oxygenation. More recently, it has become apparent that various medical interventions, including blood transfusion28 and peritoneal insufflation with carbon dioxide29 can adversely affect red blood cell NO bioactivity with resultant reductions in organ blood flow. Based on the current results, brain death may constitute an acute stress characterized by relatively rapid reductions in SNO-Hb (i.e. within 10 h), leading to decreases in oxygen utilization (Figure 2). Our data thus provide a new perspective on the peripheral response to cessation of central nervous system activity in which the sequelae represent a system-wide failure of microvascular oxygen delivery rather than a disparate collection of failing organs.
The mechanism to account for the depletion of SNO-Hb following brain death is probably mutli-fold. Increasing acidosis disfavors the binding of NO to the beta cysteine residue of Hb21. The post-brain death catecholamine surge with resultant vasoconstriction and progressive lung injury may also contribute to this decline through effects on Hb oxygenation5.
The inability of SNO-Hb-depleted red blood cells to facilitate hypoxic vasodilation has been well-documented16, 28, 30 and thus provides a potential therapeutic target. Indeed, nitrosylating therapies directed towards increasing SNO-Hb have recently been developed and have shown therapeutic potential. In particular, ENO has been shown to correct impairments in tissue perfusion in a number of clinical and preclinical conditions that have in common a deficiency in SNO-Hb16, 29, 31–34. In the current setting, inhalation of ENO following brain death restored SNO-Hb concentration, enhanced tissue oxygenation and blood flow, and reduced markers of injury and inflammation. Collectively, these measures of organ viability are predictive of post-graft function35.
Particularly notable were the effects of ENO on kidney function where 10 h after brain death no significant changes were seen in inulin clearance, GFR, or serum creatinine concentration (Figure 3). In the control group, the > 0.3 mg/dl increase in creatinine (Table 1) meets the criteria for diagnosing acute kidney injury (AKI)36. Serum creatinine can increase during donor support37 with some authors reporting > 20% elevations (a level observed in our study) in more than 40% of human donors prior to organ procurement38. Elevated donor creatinine and reduced GFR are significant risk factors for delayed graft function and graft failure in kidney transplant recipients39. The major non-drug based cause of AKI is renal hypo-perfusion40, which implies there were differences in kidney blood flow between the two experimental groups. ENO increased buccal blood flow (Figure 2), a finding that is consistent with our previous work demonstrating this agent can maintain flow to multiple organs29. As such, our data support the notion that brain death induced perturbations in blood flow to the kidneys (and to other organs) are reversed by inhalation of ENO.
Nitrosylation therapy with ENO may confer additional benefits. Hemoglobin is the major target of inhaled ENO but SNO-Hb can exchange NO with other proteins via transnitrosylation reactions to distribute NO bioactivity in vivo42. Under normal conditions, the activities of many inflammatory and pro-apoptotic proteins including toll-like receptors, interleukins, tumor necrosis factors, caspases, etc are tightly-controlled by S-nitrosylation47–49; their subsequent over-expression following loss of CNS function43–46 may well reflect a loss of this regulatory control and are thus potential targets for ENO. Indeed, administration of a nitrosylating agent (or an exogenous SNO) can produce beneficial results in a number of acute and chronic inflammatory conditions where SNO levels are reduced50–55. Since neutrophil infiltration appears to negatively impact post-graft function56, a more in-depth elucidation of ENO’s actions on the inflammatory cascade following brain death would be worthwhile. In addition, nitrosylating therapy may also provide the means to enhance the activities of protective genes such as HIF-157 and HO-158 (a more selective approach than current therapies such as using dopamine to induce HO-1 activity59).
With respect to recording blood flow and tissue oxygenation, we selected the buccal mucosa as a monitoring site because this location allowed for easy sensor placement and it provided for continued access in case of displacement. Previous studies with the T-Stat have demonstrated measurements of the buccal mucosa correlate well with recordings from more invasive locations and that changes in buccal mucosa oxygen levels and blood flow are sensitive indicators of global oxygenation status20. In addition, it has long been recognized that the external carotid arteries can remain well-perfused following brain death, even as blood flow within the internal carotids is significantly impaired41. While additional testing is needed, the current findings suggest that this site may be a convenient location to monitor for perfusion changes during donor support.
Our study has limitations. The method of brain death induction was rapid whereas in humans there can be a more delayed increase in ICP and this can subsequently alter the pattern of systemic organ disruption60. We were not in a position to conduct histologic assessments to determine if the functional benefits of ENO also prevented structural damage. The period of monitoring was short compared to the interval between declaration of death and organ procurement for some brain dead human donors. Nonetheless, it is doubtful that the observed depletion of SNO-Hb in the standard therapy control group would have resolved if the experimental duration increased. We deliberately chose to not initiate any hormone therapy so as to focus on documenting the effects of ENO inhalation. As administration of vasopressin, thyroxine, insulin, etc to human donors may confer benefits with respect to post-graft function61, it is tempting to speculate that such benefits could be augmented when NO bioactivity levels are maintained following brain death.
In summary, inhalation of the S-nitrosylating agent ENO is an effective means to attenuate brain death-induced reductions in SNO-Hb concentrations, maintain tissue oxygenation, and reduce tissue injury, inflammation, and organ damage. As a result, clinical assessments of the utility and benefits of this intervention may be warranted. More generally, the present findings further support the importance of endocrine NO bioactivity in the regulation of organ function and they provide a novel example in which aberrant S-nitrosylation can be reversed with beneficial effects. Our results suggest that ENO has potential to improve physiologic status and organ function following brain death and to possibly expand the pool of organs available for donation.
Supplementary Material
Acknowledgements
The authors wish to thank Mr. Mike Fierro from Spectros Corporation for his assistance with the analyses of the data generated by the T-Stat ischemia detector, Dr. Thomas Coffman from the Division of Nephrology at Duke University Medical Center for his counsel on renal physiology, and Dr. Stephen Vaughn from Custom Gas Solutions for his assistance with the ENO delivery device.
This work was funded by a grant from the Duke Translational Research Institute (National Institutes of Health National Center for Research Resources Clinical and Translational Science Awards Program KL2RR024127); the Duke Anesthesiology Research Fund; and the Institute for Transformative Molecular Medicine at Case Western Reserve University.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Conflicts of Interest
JDR has financial interests in Miach Medical Innovations and N30. JSS has financial interests in LifeHealth, N30, and Vindica Therapeutics. Miach is an early-stage medical device company, the others are all early stage biotech companies developing nitric oxide related technologies. These companies had no involvement in this study. There are no conflicts to report for the other co-authors.
List of Supplemental Digital Content
The supplemental digital content consists of a single file titled Yurcisin et al Supplemental Digital Content Ann Surg. The document contains additional physiologic and serum chemistry data.
References
- 1.Pratschke J, Wilhelm MJ, Kusaka M, et al. Accelerated rejection of renal allografts from brain-dead donors. Ann Surg. 2000;232(2):263–271. doi: 10.1097/00000658-200008000-00017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Wolfe RA, Roys EC, Merion RM. Trends in organ donation and transplantation in the United States: 1999–2008. Am J Transplant. 2010;10(4 Pt 2):961–972. doi: 10.1111/j.1600-6143.2010.03021.x. [DOI] [PubMed] [Google Scholar]
- 3.Mascia L, Mastromauro I, Viberti S, et al. Management to optimize organ procurement in brain dead donors. Minerva Anestesiologica. 2009;75(3):125–133. [PubMed] [Google Scholar]
- 4.Powner DJ. Brain death: compliance, consequences and care of the adult donor. Yearbook of Intensive Care and Emergency Medicine. 2007:976–985. [Google Scholar]
- 5.Doctor A, Stamler JS. NO Transport in Blood: a third gas in the respiratory cycle. Comprehensive Physiology. 2011;1 doi: 10.1002/cphy.c090009. (10.1002/cphy.c090009) [DOI] [PubMed] [Google Scholar]
- 6.Adrie C, Monchi M, Fulgencio JP, et al. Immune status and apoptosis activation during brain death. Shock. 2010;33(4):353–362. doi: 10.1097/SHK.0b013e3181b65b99. [DOI] [PubMed] [Google Scholar]
- 7.Foster MW, Hess DT, Stamler JS. Protein S-nitrosylation in health and disease: a current perspective. Trends Mol Med. 2009;15(9):391–404. doi: 10.1016/j.molmed.2009.06.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Smith M. Physiologic changes during brain stem death--lessons for management of the organ donor. J Heart Lung Transplant. 2004;23(9 Suppl):S217–S222. doi: 10.1016/j.healun.2004.06.017. [DOI] [PubMed] [Google Scholar]
- 9.Allen BW, Stamler JS, Piantadosi CA. Hemoglobin, nitric oxide and molecular mechanisms of hypoxic vasodilation. Trends Mol Med. 2009;15(10):452–460. doi: 10.1016/j.molmed.2009.08.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Stamler JS, Jia L, Eu JP, et al. Blood flow regulation by S-nitrosohemoglobin in the physiological oxygen gradient. Science. 1997;276(5321):2034–2037. doi: 10.1126/science.276.5321.2034. [DOI] [PubMed] [Google Scholar]
- 11.McMahon TJ, Moon RE, Luschinger BP, et al. Nitric oxide in the human respiratory cycle. Nat Med. 2002;8(7):711–717. doi: 10.1038/nm718. [DOI] [PubMed] [Google Scholar]
- 12.Crawford JH, Chacko BK, Pruitt HM, et al. Transduction of NO-bioactivity by the red blood cell in sepsis: novel mechanisms of vasodilation during acute inflammatory disease. Blood. 2004;104(5):1375–1382. doi: 10.1182/blood-2004-03-0880. [DOI] [PubMed] [Google Scholar]
- 13.Datta B, Tufnell-Barrett T, Bleasdale RA, et al. Red blood cell nitric oxide as an endocrine vasoregulator: a potential role in congestive heart failure. Circulation. 2004;109(11):1339–1342. doi: 10.1161/01.CIR.0000124450.07016.1D. [DOI] [PubMed] [Google Scholar]
- 14.James PE, Lang D, Tufnell-Barret T, et al. Vasorelaxation by red blood cells and impairment in diabetes: reduced nitric oxide and oxygen delivery by glycated hemoglobin. Circ Res. 2004;94(7):976–983. doi: 10.1161/01.RES.0000122044.21787.01. [DOI] [PubMed] [Google Scholar]
- 15.Liu L, Yan Y, Zeng M, et al. Essential roles of S-nitrosothiols in vascular homeostasis and endotoxic shock. Cell. 2004;116(4):617–628. doi: 10.1016/s0092-8674(04)00131-x. [DOI] [PubMed] [Google Scholar]
- 16.McMahon TJ, Ahearn GS, Moya MP, et al. A nitric oxide processing defect of red blood cells created by hypoxia: deficiency of S-nitrosohemoglobin in pulmonary hypertension. Proc Natl Acad Sci U S A. 2005;102(41):14801–14806. doi: 10.1073/pnas.0506957102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Padron J, Peiro C, Cercas E, et al. Enhancement of S-nitrosylation in glycosylated hemoglobin. Biochem Biophys Res Commun. 2000;271(1):217–221. doi: 10.1006/bbrc.2000.2617. [DOI] [PubMed] [Google Scholar]
- 18.Pawloski JR, Hess DT, Stamler JS. Impaired vasodilation by red blood cells in sickle cell disease. Proc Natl Acad Sci U S A. 2005;102(7):2531–2536. doi: 10.1073/pnas.0409876102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Swindle MM. article-title>Swine in the laboratory: surgery, anesthesia, imaging, and experimental techniques. 2 ed. Boca Raton, FL: CRC Press; 2007. [Google Scholar]
- 20.Benaron DA, Parachikov IH, Friedland S, et al. Continuous, noninvasive, and localized microvascular tissue oximetry using visible light spectroscopy. Anesthesiology. 2004;100(6):1469–1475. doi: 10.1097/00000542-200406000-00019. [DOI] [PubMed] [Google Scholar]
- 21.Hausladen A, Rafikov R, Angelo M, et al. Assessment of nitric oxide signals by triiodide chemiluminescence. Proc Natl Acad Sci U S A. 2007;104(7):2157–2162. doi: 10.1073/pnas.0611191104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.McMahon TJ, Stamler JS. Concerted nitric oxide/oxygen delivery by hemoglobin. Methods Enzymol. 1999;301:99–114. doi: 10.1016/s0076-6879(99)01073-3. [DOI] [PubMed] [Google Scholar]
- 23.Levey AS, Bosch JP, Lewis JB, et al. A more accurate method to estimate glomerular filtration rate from serum creatinine: a new prediction equation. Modification of Diet in Renal Disease Study Group. Annals of internal medicine. 1999;130(6):461–470. doi: 10.7326/0003-4819-130-6-199903160-00002. [DOI] [PubMed] [Google Scholar]
- 24.Orlando R, Floreani M, Padrini R, et al. Determination of inulin clearance by bolus intravenous injection in healthy subjects and ascitic patients: equivalence of systemic and renal clearances as glomerular filtration markers. Br J Clin Pharmacol. 1998;46(6):605–609. doi: 10.1046/j.1365-2125.1998.00824.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Marsilio R, Naturale M, Manghi P, et al. Rapid and simple determination of inulin in biological fluids by high-performance liquid chromatography with light-scattering detection. J Chromatogr B Biomed Sci Appl. 2000;744(2):241–247. doi: 10.1016/s0378-4347(00)00226-7. [DOI] [PubMed] [Google Scholar]
- 26.Roelsgaard K, Botker HE, Stodkilde-Jorgensen H, et al. Effects of brain death and glucose infusion on hepatic glycogen and blood hormones in the pig. Hepatology. 1996;24(4):871–875. doi: 10.1002/hep.510240419. [DOI] [PubMed] [Google Scholar]
- 27.Kazemeyni SM, Esfahani F. Influence of hypernatremia and polyuria of brain-dead donors before organ procurement on kidney allograft function. Urol J. 2008;5(3):173–177. [PubMed] [Google Scholar]
- 28.Reynolds JD, Ahearn GS, Angelo M, et al. S-nitrosohemoglobin deficiency: a mechanism for loss of physiological activity in banked blood. Proc Natl Acad Sci U S A. 2007;104(43):17058–17062. doi: 10.1073/pnas.0707958104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Shimazutsu K, Uemura K, Auten KM, et al. Inclusion of a nitric oxide congener in the insufflation gas repletes S-nitrosohemoglobin and stabilizes physiologic status during prolonged carbon dioxide pneumoperitoneum. Clin Transl Sci. 2009;2(6):405–412. doi: 10.1111/j.1752-8062.2009.00154.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Diesen DL, Hess DT, Stamler JS. Hypoxic vasodilation by red blood cells: evidence for an s-nitrosothiol-based signal. Circulation research. 2008;103(5):545–553. doi: 10.1161/CIRCRESAHA.108.176867. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Ali NA, Eubanks WS, Stamler JS, et al. A method to attenuate pneumoperitoneum-induced reductions in splanchnic blood flow. Ann Surg. 2005;241(2):256–261. doi: 10.1097/01.sla.0000153034.54128.5e. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Moya MP, Gow AJ, Califf RM, et al. Inhaled ethyl nitrite gas for persistent pulmonary hypertension of the newborn. Lancet. 2002;360(9327):141–143. doi: 10.1016/S0140-6736(02)09385-6. [DOI] [PubMed] [Google Scholar]
- 33.Moya MP, Gow AJ, McMahon TJ, et al. S-nitrosothiol repletion by an inhaled gas regulates pulmonary function. Proc Natl Acad Sci U S A. 2001;98(10):5792–5797. doi: 10.1073/pnas.091109498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Sheng H, Reynolds JD, Auten RL, et al. Pharmacologically augmented S-nitrosylated hemoglobin improves recovery from murine subarachnoid hemorrhage. Stroke. 2011;42(2):471–476. doi: 10.1161/STROKEAHA.110.600569. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Wood KE, McCartney J. Management of the potential organ donor. Transplantation Reviews. 2007;21:204–218. [Google Scholar]
- 36.Mehta RL, Kellum JA, Shah SV, et al. Acute Kidney Injury Network: report of an initiative to improve outcomes in acute kidney injury. Crit Care. 2007;11(2):31. doi: 10.1186/cc5713. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Blasco V, Leone M, Antonini F, et al. Comparison of the novel hydroxyethylstarch 130/0.4 and hydroxyethylstarch 200/0.6 in brain-dead donor resuscitation on renal function after transplantation. British Journal of Anaesthesia. 2008;100(4):504–508. doi: 10.1093/bja/aen001. [DOI] [PubMed] [Google Scholar]
- 38.Blasco V, Leone M, Bouvenot J, et al. Impact of intensive care on renal function before graft harvest: results of a monocentric study. Crit Care. 2007;11(5):R103. doi: 10.1186/cc6120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Moore J, Tan K, Cockwell P, et al. Predicting early renal allograft function using clinical variables. Nephrol Dial Transplant. 2007;22(9):2669–2677. doi: 10.1093/ndt/gfm249. [DOI] [PubMed] [Google Scholar]
- 40.Sharfuddin AA, Molitoris BA. Pathophysiology of ischemic acute kidney injury. Nat Rev Nephrol. 2011;7(4):189–200. doi: 10.1038/nrneph.2011.16. [DOI] [PubMed] [Google Scholar]
- 41.Bradac GB, Simon RS. Angiography in brain death. Neuroradiology. 1974;7(1):25–28. doi: 10.1007/BF00344671. [DOI] [PubMed] [Google Scholar]
- 42.Stamler JS, Reynolds JD, Hess DT. Endocrine nitric oxide bioactivity and hypoxic vasodilation by inhaled nitric oxide. Circ Res. 2012;110(5):652–654. doi: 10.1161/CIRCRESAHA.111.263996. [DOI] [PubMed] [Google Scholar]
- 43.Mas VR, Archer KJ, Yanek K, et al. Gene expression patterns in deceased donor kidneys developing delayed graft function after kidney transplantation. Transplantation. 2008;85(4):626–635. doi: 10.1097/TP.0b013e318165491f. [DOI] [PubMed] [Google Scholar]
- 44.Nijboer WN, Schuurs TA, van der Hoeven JAB, et al. Effect of brain death on gene expression and tissue activation in human donor kidneys. Transplantation. 2004;78(7):978–986. doi: 10.1097/01.tp.0000135565.49535.60. [DOI] [PubMed] [Google Scholar]
- 45.de Jonge J, Kurian S, Shaked A, et al. Unique Early Gene Expression Patterns in Human Adult-to-Adult Living Donor Liver Grafts Compared to Deceased Donor Grafts. American Journal of Transplantation. 2009;9(4):758–772. doi: 10.1111/j.1600-6143.2009.02557.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Lopez SP, Moreno NV, Augusto DE, et al. A Molecular Approach to Apoptosis in the Human Heart During Brain Death. Transplantation. 2008;86(7):977–982. doi: 10.1097/TP.0b013e318186d6d6. [DOI] [PubMed] [Google Scholar]
- 47.Hess DT, Stamler JS. Regulation by S-nitrosylation of Protein Posttranslational Modification. J Biol Chem. 2011 doi: 10.1074/jbc.R111.285742. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Seth D, Stamler JS. The SNO-proteome: causation and classifications. Curr Opin Chem Biol. 2011;15(1):129–136. doi: 10.1016/j.cbpa.2010.10.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Hess DT, Matsumoto A, Kim SO, et al. Protein S-nitrosylation: purview and parameters. Nat Rev Mol Cell Biol. 2005;6(2):150–166. doi: 10.1038/nrm1569. [DOI] [PubMed] [Google Scholar]
- 50.Auten RL, Mason SN, Whorton MH, et al. Inhaled ethyl nitrite prevents hyperoxia-impaired postnatal alveolar development in newborn rats. Am J Respir Crit Care Med. 2007;176(3):291–299. doi: 10.1164/rccm.200605-662OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Prasad R, Giri S, Nath N, et al. GSNO attenuates EAE disease by S-nitrosylation-mediated modulation of endothelial-monocyte interactions. Glia. 2007;55(1):65–77. doi: 10.1002/glia.20436. [DOI] [PubMed] [Google Scholar]
- 52.Savidge TC, Newman P, Pothoulakis C, et al. Enteric glia regulate intestinal barrier function and inflammation via release of S-nitrosoglutathione. Gastroenterology. 2007;132(4):1344–1358. doi: 10.1053/j.gastro.2007.01.051. [DOI] [PubMed] [Google Scholar]
- 53.Marshall HE, Potts EN, Kelleher ZT, et al. Protection from lipopolysaccharide-induced lung injury by augmentation of airway S-nitrosothiols. Am J Respir Crit Care Med. 2009;180(1):11–18. doi: 10.1164/rccm.200807-1186OC. [DOI] [PMC free article] [PubMed] [Google Scholar] [Research Misconduct Found]
- 54.Que LG, Liu L, Yan Y, et al. Protection from experimental asthma by an endogenous bronchodilator. Science. 2005;308(5728):1618–1621. doi: 10.1126/science.1108228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Snyder AH, McPherson ME, Hunt JF, et al. Acute effects of aerosolized S-nitrosoglutathione in cystic fibrosis. Am J Respir Crit Care Med. 2002;165(7):922–926. doi: 10.1164/ajrccm.165.7.2105032. [DOI] [PubMed] [Google Scholar]
- 56.Kim IK, Bedi DS, Denecke C, et al. Impact of innate and adaptive immunity on rejection and tolerance. Transplantation. 2008;86(7):889–894. doi: 10.1097/TP.0b013e318186ac4a. [DOI] [PubMed] [Google Scholar]
- 57.Palmer LA, Gaston B, Johns RA. Normoxic stabilization of hypoxia-inducible factor-1 expression and activity: redox-dependent effect of nitrogen oxides. Mol Pharmacol. 2000;58(6):1197–1203. doi: 10.1124/mol.58.6.1197. [DOI] [PubMed] [Google Scholar]
- 58.Foresti R, Clark JE, Green CJ, et al. Thiol compounds interact with nitric oxide in regulating heme oxygenase-1 induction in endothelial cells. Involvement of superoxide and peroxynitrite anions. J Biol Chem. 1997;272(29):18411–18417. doi: 10.1074/jbc.272.29.18411. [DOI] [PubMed] [Google Scholar]
- 59.Ollinger R, Pratschke J. Role of heme oxygenase-1 in transplantation. Transpl Int. 2010;23(11):1071–1081. doi: 10.1111/j.1432-2277.2010.01158.x. [DOI] [PubMed] [Google Scholar]
- 60.Singhal AK, Sheng X, Drakos SG, et al. Impact of donor cause of death on transplant outcomes: UNOS registry analysis. Transplant Proc. 2009;41(9):3539–3544. doi: 10.1016/j.transproceed.2009.06.192. [DOI] [PubMed] [Google Scholar]
- 61.Ranasinghe AM, Bonser RS. Endocrine changes in brain death and transplantation. Best Pract Res Clin Endocrinol Metab. 2011;25(5):799–812. doi: 10.1016/j.beem.2011.03.003. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.