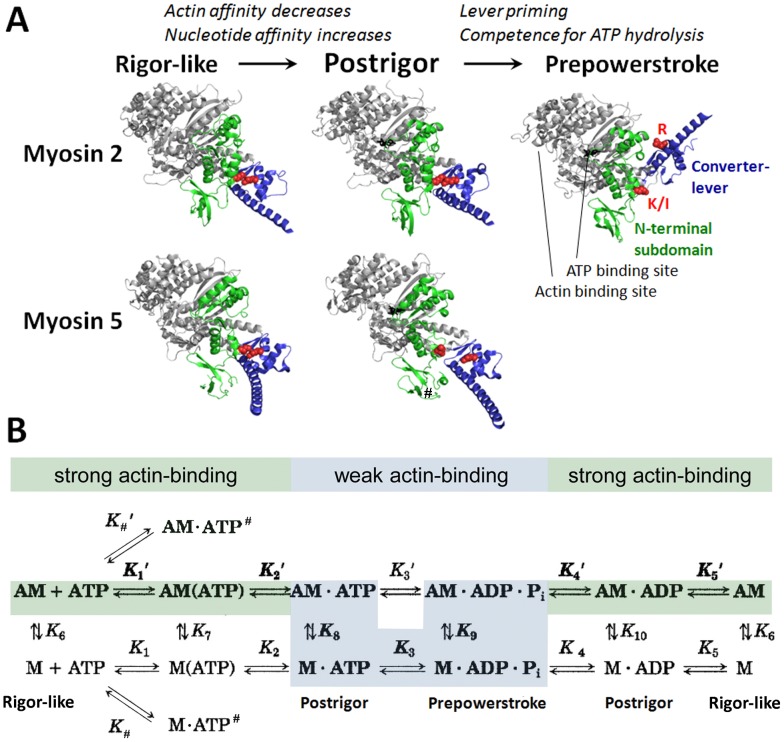
Figure 1. Structural and kinetic transitions of myosin.
A, Nucleotide-induced structural changes of the myosin MD visualized by crystal structures of myosins 2 (upper row) and 5 (lower row) in the rigor-like, postrigor and prepowerstroke conformations (PDB codes: 3I5G, Loligo pealei (Lp) myosin 2 rigor-like; 3I5F, Lp myosin 2 postrigor; 1QVI, Argopecten irradians (Ai) myosin 2 prepowerstroke; 1OE9, Gallus gallus (Gg) m5a rigor-like; 1W7J, Gg m5a postrigor). The NTS (including the SH3-like domain) and the converter (including the proximal part of the lever) are shown in green and blue, respectively. Amino acid residues involved in the subdomain interaction investigated in this study are shown in red (K81 (Lp and Ai myosin 2) or I67 (Gg m5a) of the NTS, labeled ’K/I’; and R721 (Lp myosin 2) or R719 (Ai myosin 2) or R710 (Gg m5a) of the converter, labeled ’R’). Note that the separation of this residue pair occurs upon the postrigor-prepowerstroke transition in myosin 2, whereas it occurs upon the rigor-postrigor transition in m5a. Bound nucleotides are shown as black sticks. Light chains are omitted for clarity. See also Table S1 for structural details. B, Kinetic scheme of the actomyosin mechanoenzymatic cycle, with the assignment of identified actin-detached myosin conformations. Upper and lower rows indicate enzymatic steps occurring in actin-attached (AM) and detached (M) forms of myosin, respectively. The main flux pathway is indicated by shading and bold characters. Strong and weak actin-binding states are shaded in green and cyan, respectively. In the main flux pathway, ATP binding to actomyosin occurs in two steps (a collision step (K 1’) and a subsequent isomerization (K 2’)). The myosin MD then rapidly dissociates from actin (K 8). The K 3 step involves the postrigor-prepowerstroke conformational transition and the chemical ATP hydrolysis step, which were not resolved kinetically in this study. Following ATP hydrolysis, M.ADP.Pi rebinds to actin (K 9), and products are released (Pi in K 4’ and ADP in K 5’). Kinetic data (Fig. 2, Fig. S1, Table S2) indicate that I67K-m5aS1 and acto-I67K-m5aS1 can reversibly form an off-pathway ATP binding intermediate (M.ATP# and AM.ATP#, respectively). Arrows for associating and dissociating species are omitted for clarity. All rate and equilibrium constants are defined in the rightward and downward directions. K # and K #’ are defined as dissociation constants. A, actin; M, myosin; Pi, phosphate.