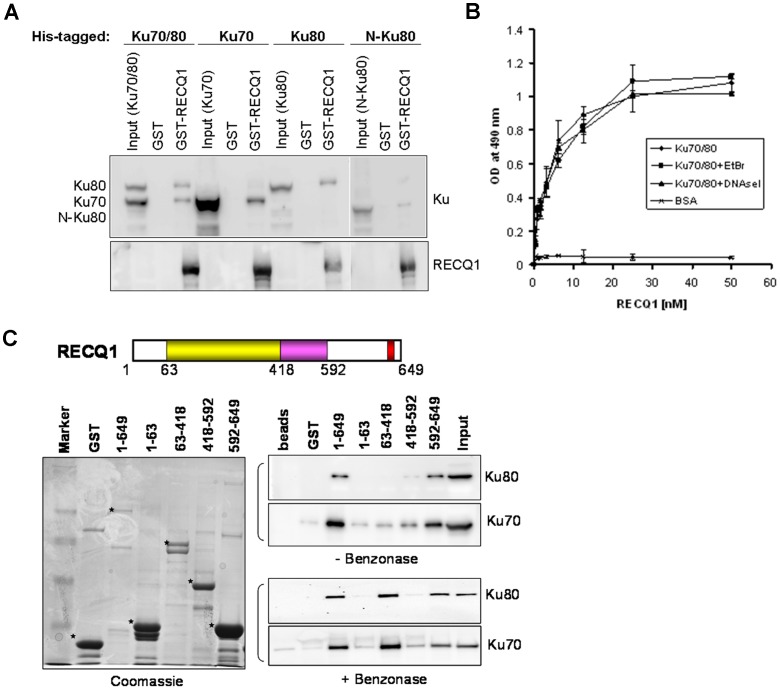
Figure 2. A direct physical interaction between RECQ1 and Ku70/80 in vitro.
A. RECQ1 directly interacts with Ku70 and Ku80. GST or GST fused-full length RECQ1 was incubated with bacterially expressed Ku70/80, Ku70, Ku80 or N-Ku80 (lacking the C-terminus amino acid residues 565–732) followed by extensive washing of the beads, SDS-PAGE, and Western transfer. The blots were probed separately with anti-His (for Ku detection) and anti-RECQ1 antibodies. Input lanes account for 10% of the bacterial lysate expressing Ku protein used in the pull-down reactions. B. Recombinant RECQ1 and Ku70/80 proteins interact directly as shown by ELISA. Either BSA or purified recombinant Ku70/80 was coated onto microtiter plates. Following blocking with 3% BSA, appropriate wells were incubated with the indicated concentrations of recombinant RECQ1 (0–50 nM) for 1 h at 30°C. Parallel wells contained DNaseI (100 U/ml) or EtBr (50 µg/ml) in the binding step to test for DNA-mediated protein interaction. Following washing, Ku70/80-bound RECQ1 was detected by ELISA using anti-RECQ1 antibody. The values represent the mean of three independent experiments performed in duplicate with SD indicated by error bars. C. GST alone or GST-RECQ1 fragments (as indicated) bound to glutathione beads were incubated overnight at 4°C with HeLa extract (500 µg) that was either untreated or pre-treated with benzonase. After extensive washings, the bound Ku70/80 was eluted with SDS sample buffer and analyzed by Western blot using anti-Ku70 and anti-Ku80 antibodies (right). Coomassie staining of the eluted proteins was done to test expression of various GST-fusion fragments of RECQ1 (left). GST-RECQ1 proteins are marked by asterisk. Marker, protein molecular weight marker.