Abstract
During systemic infection, inflammatory cytokines such as interleukin (IL)-6 are produced in excess in the brain of aged mice and induce severe behavioral deficits. However, no studies have examined how pro-inflammatory IL-6 trans-signaling is involved in the exaggerated production of IL-6 in the aged brain, nor the extent to which IL-6 trans-signaling affects other markers of neuroinflammation, adhesion molecules, and behavior. Therefore, this study investigated in aged mice the presence of IL-6 signaling subunits in microglia; the central effects of soluble gp130 (sgp130)—a natural inhibitor of the IL-6 trans-signaling pathway—on IL-6 production in microglia; and the effects of sgp130 given intracerebroventricularly (ICV) on neuroinflammation and sickness behavior caused by i.p. injection of lipopolysaccharide (LPS). Here we show that microglia isolated from aged mice have higher expression of IL-6 receptor (IL-6R) compared to microglia from adults; and the level of mRNA for ADAM17, the enzyme responsible for shedding membrane-bound IL-6R in trans-signaling, is higher in the hippocampus of aged mice compared to adults. Additionally, we show in aged mice that peripheral LPS challenge elicits a hyperactive IL-6 response in microglia, and selective blockade of trans-signaling by ICV injection of sgp130 mitigates this. The sgp130-associated inhibition of IL-6 was paralleled by amelioration of exaggerated and protracted sickness behavior in aged mice. Taken together, the results show that microglia are important regulators of the IL-6 trans-signaling response in the aged brain and sgp130 exerts an anti-inflammatory effect by inhibiting the pro-inflammatory arm of IL-6 signaling.
Keywords: Aging, microglia, interleukin-6, trans-signaling, ADAM17, sickness behavior
Introduction
Interleukin-6 receptor signaling is facilitated through two related pathways termed classical and trans-signaling (Rose-John, 2003). The receptor consists of two subunits: the IL-6 receptor-alpha chain (IL-6R), which binds IL-6, and the trans-membrane signaling subunit, glycoprotein 130 (gp130), which is the intracellular signal transducer that is constitutively expressed. Classical activation consists of the IL-6 binding to the membrane-bound IL-6R, while trans-signaling is the ability of a soluble IL-6R (sIL-6R) to bind IL-6 in the extracellular compartment to form an IL-6/sIL-6R complex and in turn, activate gp130. On the one hand, the expression pattern of membrane-bound IL-6R is limited to cells of the immune system and sparsely dispersed among other cell types (Schobitz et al., 1993). On the other hand, gp130 is ubiquitously expressed; therefore IL-6 trans-signaling confers IL-6 responsiveness in any cell type that expresses gp130 and is exposed to the IL-6/sIL-6R complex (Jones et al., 2005; Kishimoto et al., 1992). Recent studies of monocytes show that two specific members of the ADAM (a disintegrin and metalloprotease domain) family, ADAM17 and ADAM10, contribute to IL-6R shedding, which is necessary to generate sIL-6R (Matthews et al., 2003).
Although both pro-inflammatory and anti-inflammatory actions have been reported for IL-6 (Scheller et al., 2011), the trans-signaling pathway is pro-inflammatory and has been implicated in several chronic inflammatory diseases including chronic inflammatory bowel disease, rheumatoid arthritis, and colon cancer (Rose-John et al., 2006). In the brain, neurons do not express considerable amounts of IL-6R, however, they do express copious amounts of gp130 (Burton et al., 2011; Schobitz et al., 1993), alluding to the potential importance of IL-6 trans-signaling in neuroinflammation. Importantly, in the brain of old but otherwise healthy mice, microglial cells show a predilection for being activated (Godbout and Johnson, 2004; Ye and Johnson, 1999b, 2001). In this state, they constitutively express higher levels of inflammatory cytokines and are hypersensitive to signals from the peripheral immune system. The increased production of inflammatory cytokines by microglia is widely viewed to be involved in the severe sickness behavior seen in older subjects with a peripheral infection (Abraham and Johnson, 2009; Godbout et al., 2005; Henry et al., 2009). Interestingly, IL-6 was one of the first inflammatory cytokines shown to be excessively produced by microglia in the senescent brain (Ye and Johnson, 1999b) and a recent study suggests increased IL-6 trans-signaling in the brain of aged mice during peripheral infection (Burton and Johnson, 2011). However, how microglia respond upon activation of the IL-6 trans-signaling pathway is not well known nor is the contribution of trans-signaling to age-related neuroinflammation and sickness behavior. Therefore, the goal of the present study was to determine the role of IL-6 trans-signaling in neuroinflammation and sickness behavior in aged mice during a peripheral immune stimulation.
Materials and Methods
Animals and Surgery
Male BALB/c mice were obtained from our in-house breeding colony. The median life span for BALB/c mice is approximately 26 months (Morley and Trainor, 2001); therefore 3- to 6-month-old (adult) and 20- to 24-month-old (aged) male mice were used. Mice were housed in polypropylene cages and maintained at 21°C under a reverse-phase 12-h light-dark cycle with ad libitum access to water and rodent chow. At the end of each study, mice were examined post mortem for gross signs of disease (e.g., tumors or splenomegaly). Data from mice determined to be unhealthy were excluded from the analysis (<5%).
Intracerebroventricular (ICV) cannulation was performed under aseptic conditions as described previously (Abraham et al., 2008). In brief, mice were deeply anesthetized with an intraperitoneal (i.p.) injection of ketamine and xylazine (100 and 10 mg/kg, respectively) and the surgical site was shaved and sterilized. They were positioned in a stereotaxic instrument (David Kopf Instruments, Tujunga, CA) so that the frontal and parietal bones of the skull were parallel to the surgical platform. An incision roughly 1.5 cm in length was made on the cranium to reveal the bregma and a 26-gauge stainless steel cannula (Plastics One, Roanoke, VA) was placed in the right lateral cerebral ventricle according to predetermined stereotaxic coordinates (lateral 1.6 mm and antero-posterior 1 mm to the bregma, and horizontal 2 mm from the dura mater). The cannula was secured using two adjacent stainless steel screws and cranioplastic cement (Plastics One, Roanoke, VA). A dummy cannula (Plastics One, Roanoke, VA) was inserted in the guide cannula to prevent occlusion and infection. Mice were injected subcutaneously with buprenorphine (0.05 mg/kg) following surgery and then again 8–12 h later to aid with any post-operative discomfort. Mice were provided a minimum of 7 days to recover before any treatment or behavioral test. Accurate placement of the cannula was confirmed by allowing 2 μl of sterile saline to flow via gravity into the lateral ventricle. If cannula placement could not be confirmed by gravity flow the animal was excluded from the study. All procedures were in accordance with the guidelines for the care and use of laboratory animals and were approved by the University of Illinois Institutional Animal Care and Use Committee.
Experimental protocols
Mice were routinely handled 1–2 min each day for 7 days before experimentation to acclimate them to handling. Animals were injected ICV with sterile saline containing 0.1% BSA (vehicle) or 100 ng sgp130 (R&D systems, Minneapolis, MN) dissolved in 2 μl vehicle. At the same time as the ICV injection, mice were injected i.p. with sterile saline or 0.33 mg/kg BW (10 μg) LPS (serotype 0127:B8, obtained from Sigma, St. Louis, MO).
To measure changes in cytokines, mice not exposed to the behavior paradigms were injected ICV with vehicle or sgp130 (100 ng) and i.p. with vehicle or LPS (10 μg), and killed 6 h later by CO2 asphyxiation. The brain was rapidly removed and dissected to obtain hippocampal tissue which was snap frozen in liquid nitrogen and stored at −80°C until later analysis.
Assessment of Sickness
Mice were maintained in their home cage and locomotor activity was video recorded during 5-min periods using a camera mounted directly above the center of the cage floor. Behavioral tests were conducted during the dark phase (between 07:00 and 19:00) of the light/dark cycle under infrared lighting. Baseline behavior was taken just before treatment (0 h) and behavior was recorded 4, 6, 8, and 24 h after administration of sgp130 (0 ng or 100 ng ICV) and LPS (0 μg or 10μg i.p.). Animal movement was tracked from videos by Ethovision (Noldus, Leesburg, VA) software, to determine total distance moved. Body weight and food intake were measured at each time point over the 24-h period.
Microglia isolation
In experiments for flow cytometry, microglia from whole brain were isolated as described previously, with few modifications (Henry et al., 2009). Mice were euthanized by CO2 asphyxiation and whole brains were collected and stored in sterile PBS. Brains were homogenized by passage through a 70 μm cell strainer in Dulbecco’s Phosphate Buffered Salt Solution (DPBS) supplemented with 0.2% glucose. Resulting homogenates were centrifuged at 600 × g for 6 min at 10°C. Supernatants were removed and cell pellets were re-suspended in a 70% isotonic Percoll (GE-healthcare, Uppsala, Sweden) supplemented with phenol red (0.01%) at room temperature. A discontinuous Percoll density gradient of 70%, 50%, 35%, and 0% isotonic Percoll was set up. The gradient was centrifuged for 20 min at 2000 × g and microglia were collected from the interphase between the 70% and 50% Percoll layers (Frank et al., 2006). Cells were washed with DPBS and then re-suspended in PBS- 0.5% BSA/0.01% sodium azide solution (flow buffer). The number of viable cells was determined using a hemacytometer and 0.1% trypan blue; each isolation yielded approximately 3 ×105 viable microglia.
Extracellular and intracellular flow cytometric analysis
Flow cytometric analysis of microglial surface and intracellular markers was performed based on BD Cytofix/Cytoperm Plus fixation/permeabilization protocol (BD biosciences, CA), as described previously, with a few modifications (Henry et al., 2009). In brief, cells isolated by Percoll density gradient were incubated in DMEM (Bio-Whittaker, Cambrex, MD) supplemented with 10% FBS (Hyclone, Logan, UT), 200 mM glutamine, and 100 units/ml penicillin/streptomycin (Invitrogen, Carlsbad, CA), and a solution containing brefeldin A (BD Biosciences, CA) at 37°C in a humidified incubator under 5% CO2, for 4 h. Cells were then washed in flow buffer and Fc receptors were blocked with anti-CD16/CD32 antibody (eBioscience, CA). In initial extracellular experiments, microglia were incubated with anti-CD11b-PE-Cy7, anti-CD45-FITC, anti-CD126 (IL-6Rα)-PE, and anti-CD130 (gp130)-APC (eBioscience, CA). For intracellular cytokine experiments, microglia were first incubated with anti-CD11b-APC, anti-CD45-FITC, and anti-MHC-II-PE antibodies (eBioscience, CA). Next, cells were fixed and permeabilized with BD Cytofix/Cytoperm™ solution for 20 min. Microglia were washed with BD Perm/Wash™ buffer, re-suspended in BD Perm/Wash™ buffer and incubated with anti-IL-6-PE (eBioscience, CA) for 30 min. Cells were washed twice in BD Perm/Wash™ buffer and re-suspended in flow buffer. Expression of surface and intracellular antigens was determined using a Becton-Dickinson LSR II Flow Cytometer (Red Oaks, CA). Thirty thousand events were collected, microglia were identified by CD11b+ and CD45+low expression (Ford et al., 1995). Gating was determined based on fluorescently labeled isotype antibodies for PE-Cy7, FITC, PE, APC (eBioscience, San Diego, CA), and unstained samples as controls. Flow data were analyzed using FCS Express software (De Novo Software, Los Angeles, CA).
Cytokine mRNA measurement by quantitative real-time PCR
Total RNA from hippocampal tissue was isolated using the Tri Reagent protocol (Sigma, St. Louis, MO). A QuantiTect Reverse Transcription Kit (Qiagen, Valencia, CA) was used for cDNA synthesis with integrated removal of genomic DNA contamination according to the manufacturer’s protocol. Quantitative real-time PCR was performed using the Applied Biosystems (Foster, CA) Assay-on Demand Gene Expression protocol as previously described (Krzyszton et al., 2008). In brief, cDNA was amplified by PCR where a target cDNA (IL-6, Mm00446190_m1; IL-1β, Mm00434228_m1; ADAM10 Mm00545742_m1; ADAM17 Mm00456428_m1; Icam Mm00516023_m1; Vcam Mm01320970_m1; Socs1 Mm00782550_s1; Socs3 Mm00545913_s1; iNOS2 Mm01309902) and a reference cDNA (glucose-3 phosphate dehydrogenase, Mm99999915_g1) were amplified simultaneously using an oligonucleotide probe with a 5′ fluorescent reporter dye (6-FAM) and a 3′ quencher dye (NFQ). PCR reactions were performed in triplicate under the following conditions: 50°C for 2 min, 95°C for 10 min, followed by 40 cycles of 95°C for 15 sec, and 60°C for 1 min. Fluorescence was determined on an ABI PRISM 7900HT-sequence detection system (Perkin Elmer, Forest City, CA). Data were analyzed using the comparative threshold cycle (Ct) method, and results are expressed as fold difference.
Statistical analysis
Data were analyzed using Statview and Statistical Analysis System software (SAS Inst., Cary, NC). All data were subjected to a univariate analysis to ensure normality. Locomotor data were subjected to a 3-way ANOVA (sgp130 x LPS x time) using repeated measures in which time (0, 4, 6, 8, and 24 h) was a within subjects measure, and sgp130 (vehicle or 100 ng/mouse) and LPS (sterile saline or LPS 10 μg/mouse) were between subjects measures. All other data were subjected to one-way (age), two-way (sgp130 x LPS), or three-way (age x sgp130 x LPS) ANOVA as appropriate. Post hoc Student’s t test of least square means was used to determine if treatment means were significantly different from one another (p<0.05). All data are presented as mean ± SEM.
Results
IL-6R and gp130 expression by microglia from adult and aged mice
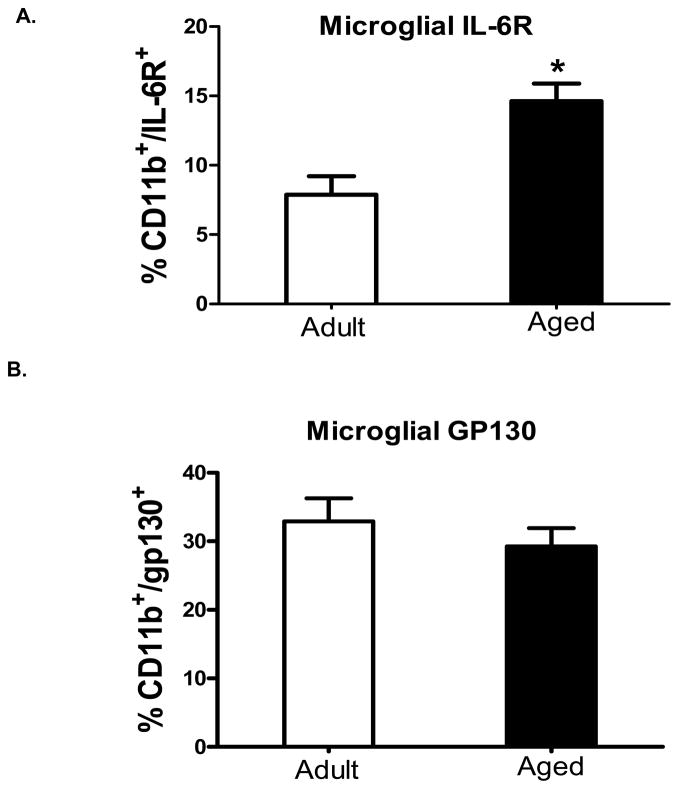
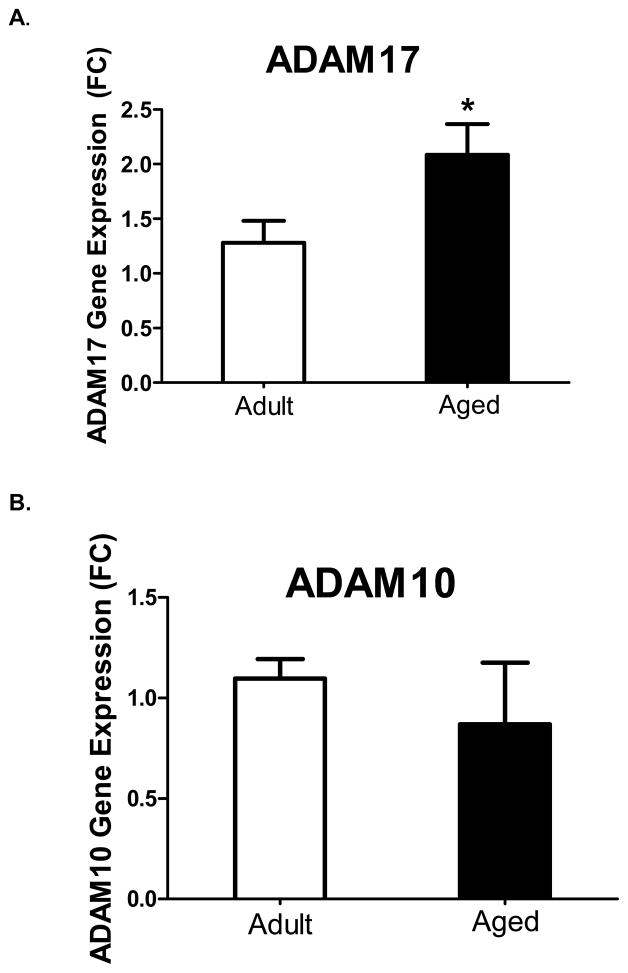
In aged mice, excessive production of IL-6 in the brain during peripheral infection contributes to behavioral pathology and may be related to dysfunctional IL-6 signaling. Thus, in an initial study we sought to determine the effects of aging on several components necessary for classical and trans-signaling by determining the expression of IL-6R and gp130 on microglia from adult and aged mice. Microglia identified as CD11b+ and CD45low were stained for IL-6R and gp130. Figure 1A and 1B, show the percentages of microglia that were CD11b+/IL-6R+ and CD11b+/gp130+, respectively. A greater proportion of microglia from aged mice expressed IL-6R compared to adults (p<0.05), while there was no difference in the expression of gp130. Hippocampal tissue, which presumably included microglia, astrocytes and neurons, was collected and mRNAs for ADAM17 and ADAM10 were measured (Figure 2). Aged animals had a higher baseline level of ADAM17 mRNA (Figure 2A; p<0.05), while there was no difference in ADAM10 mRNA (Figure 2B). Coincidently, a recent study has shown that ADAM17 but not ADAM10 is a requisite for IL-6R shedding (Chalaris et al., 2010). Taken together, these data suggest (1) microglia in aged mice may be more responsive to IL-6 via the classical signaling pathway due to an up regulation of the IL-6R; and/or (2) aged mice may produce more sIL-6R in the brain—a prerequisite for central IL-6 trans-signaling—due to an up regulation of IL-6R and ADAM17.
Figure 1. Differential expression of IL-6 receptor and gp130 on microglia isolated from adult and aged mice.
Average percentage of cells that were A) CD11b+/IL-6R+ B) and CD11b+/gp130+. Cell surface markers were assessed for CD11b, CD45, and IL-6R or gp130 expression; compared with isotype and unstained controls. Bars represent the means ± SEM (n = 8). Means marked with a * are significantly different from each other (P < 0.05).
Figure 2. Hippocampal gene expression of IL-6 receptor sheddases ADAM17 and ADAM10 from adult and aged mice.
Hippocampal tissue was collected and assayed for A) ADAM17 and B) ADAM10. Aged animals had a significant upregulation of baseline levels of ADAM17 gene expression. Bars represent the mean ± SEM (n = 8–9) Means with * are statistically different from each other (P<0.05).
sgp130 inhibits LPS-induced sickness behavior in aged mice
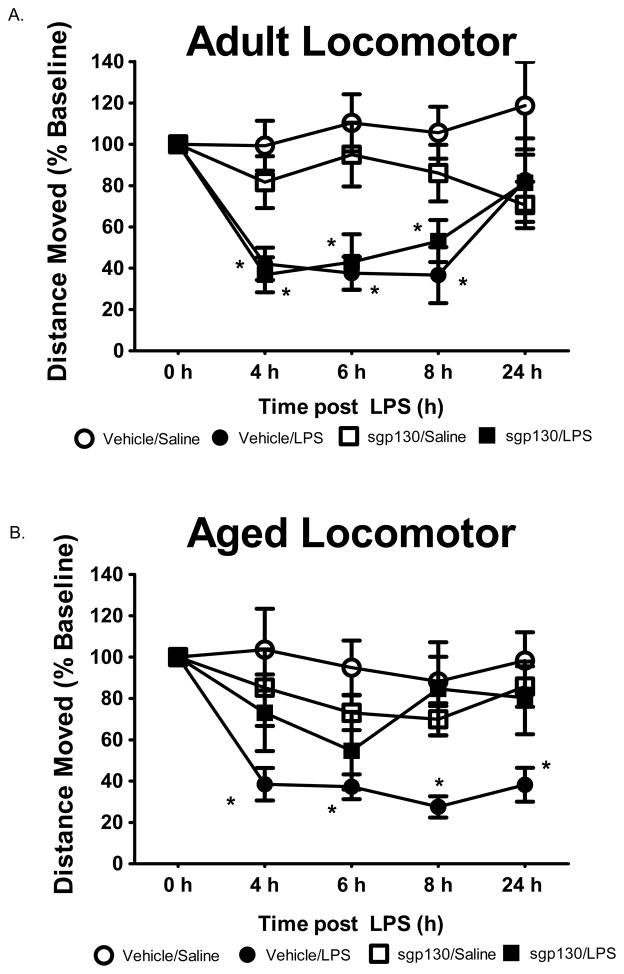
Previous studies in IL-6 knockout mice and in wild type mice injected with sgp130 suggest IL-6 trans-signaling has a role in behavioral changes caused by peripheral infection (Burton et al., 2011; Nguyen et al., 2011). Another study involving aged mice suggested that increased IL-6 trans-signaling inhibits consolidation of memories that are hippocampal dependent (Burton and Johnson, 2011). However, if IL-6 trans-signaling is involved in the exacerbated behavioral response induced by infection in older mice is not known. Therefore, we next investigated the effects of centrally administered sgp130 on sickness behavior induced by i.p. injection of LPS. In separate but similar studies, adult and aged mice were administered sgp130 ICV and LPS i.p. and spontaneous locomotor behavior, food intake and body weight were assessed as measures of sickness. In adult mice, figure 3A shows locomotor behavior was depressed by LPS from 4 to 8 h post injection but returned to normal by 24 h (LPS x time, [F (2,38) = 6.64, p < 0.001]). Treatment of adult mice with sgp130 did not influence the effects of LPS on locomotor behavior (Figure 3A, LPS x sgp130, [F (2, 38) = 2.47, p > 0.10]). In aged mice, however, a sgp130 x LPS interaction affected locomotor behavior (Figure 3B, [F (2, 44) = 5.13, p < 0.01]). Whereas aged mice given LPS alone had depressed locomotor behavior throughout the entire 24 h period, in those given sgp130 in conjunction with LPS, locomotor behavior was fully restored by 8 h. Treatment effects on food intake and body weight in adult and aged mice are presented in Table 1. Consistent with locomotor activity, sgp130 given ICV protected aged mice from the effects of LPS given i.p. As sgp130 prevents the IL-6-sIL-6R from activating intracellular gp130, these data suggest that an increase in IL-6 trans-signaling in the brain of aged mice contributes to the severe behavioral deficits seen during infection.
Figure 3. sgp130-mediated LPS-induced sickness behavior in adult and aged animals.
Mice were injected ICV with vehicle or sgp130 (100ng) and i.p. with sterile saline or LPS (10 μg). Spontaneous locomotor baseline behavior was measured before injections (0) and at 4, 6, 8, and 24 h post-injection. Bars represent the mean ± SEM (n = 10–11) Means with * are statistically different (P<0.05) from saline controls.
Table 1.
Weight and Food Intake 24 h after sgp130 and LPS.
| Vehicle (ICV) | sgp130 (100 ng) (ICV) | p-value | |||||
|---|---|---|---|---|---|---|---|
|
| |||||||
| Saline (i.p.) | LPS (10 μg) (i.p.) | Saline (i.p.) | LPS (10μg) (i.p.) | sgp130 | LPS | sgp130 × LPS | |
| Adult | |||||||
| Food intake (g/d) | 6.86± 0.62 | 4.18± 0.20 | 7.65± 0.36 | 3.88± 0.61 | 0.773 | <0.001* | 0.917 |
| Δ Body wt. (g) | −0.33± 0.10 | −2.56± 0.19 | −0.26± 0.10 | −2.18± 0.25 | 0.632 | <0.001* | 0.861 |
|
| |||||||
| Aged | |||||||
| Food intake (g/d) | 6.89± 0.89 | 3.43± 0.145 | 7.14± 0.33 | 5.11± 0.47 | 0.868 | 0.002* | 0.039* |
| Δ Body wt. (g) | −0.56± 0.13 | −3.40± 0.25 | −0.23± 0.33 | −2.79± 0.36 | 0.592 | <0.0001* | 0.041* |
a significant difference per treatment group
sgp130 inhibited LPS-induced microglia production of IL-6
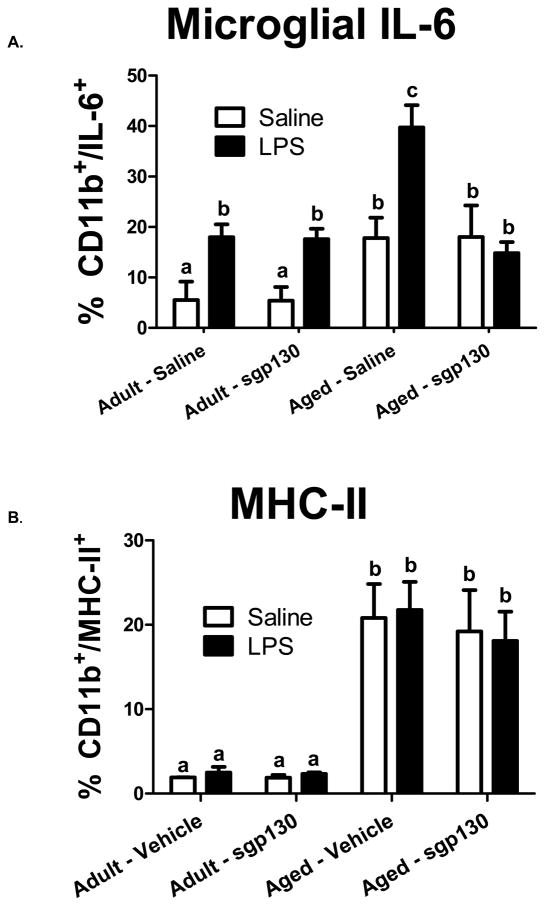
To assess the role of IL-6 trans-signaling in LPS-induced IL-6 production by microglia, we next determined intracellular IL-6 protein expression in microglia isolated from adult and aged mice 6 h after administration of sgp130 (ICV) and LPS (i.p.). Figure 4A shows the percentage of microglia from adult and aged mice stained with anti-CD11b-PE-Cy7 and intracellular anti-IL-6-PE. An age x LPS x sgp130 interaction affected microglial IL-6, [F (3, 61) = 4.07, p < 0.01]. Peripheral LPS increased the percentage of IL-6+ microglia (CD11b+/IL-16+) in both age groups. However, the LPS-induced increase in IL-6+ microglia was greater in aged mice compared to adults [F (2, 61) = 3.24, p < 0.05], an observation consistent with earlier findings (Ye and Johnson, 1999a). Figure 4A further shows that ICV sgp130 attenuated IL-6 production by microglia from aged mice, reducing the level to that seen in adults. Thus, while sgp130 did not blunt LPS-induced expression of IL-6 in aged microglia to levels seen in adult microglia, it did curtail IL-6 expression to a level consistent with the LPS-induced behavioral changes seen in similarly treated mice, suggesting that IL-6 trans-signaling plays a critical role in the exaggerated behavioral deficits observed in aged animals.
Figure 4. sgp130 ameliorates LPS-induced microglial IL-6 production in the aged, while unaltering MHC-II expression.
Adult and aged mice were injected ICV with vehicle or sgp130 (100ng) and i.p. with sterile saline or LPS (10 μg) and microglia were isolated by percoll density gradient 6 h later. Cells were subjected to the BD Cytofix/Cytoperm™ fixation/permeabilization protocol and stained with anti-CD11b-APC, anti-CD45-FITC, and anti-IL-6-PE or MHC-II-PE. Bars represent the means ± SEM (n =7–8). Means with different letters are statistically different (P<0.05) from each other.
Microglia isolated from adult and aged brains were also stained with antibodies for MHC-II and analyzed by flow cytometry. Figure 4B shows the percentage of CD11b+/MHC-II+ cells. As reported previously (Henry et al., 2009), microglia isolated from aged mice had higher MHC-II expression (CD11b+/MHC II+) compared to microglia isolated from adults (p < 0.05). However, ICV treatment with sgp130 did not affect MHC II protein on microglia.
Exaggerated IL-6 and MHC-II gene expression is modified by sgp130
To gain a broader view of the role of IL-6 trans-signaling in neuroinflammation, mRNA for several markers of inflammation (IL-1β, IL-6, MHC-II), oxidative stress (iNOS2), cytokine regulation (SOCS1 and SOCS3), cytokine modulation (ADAM10 and ADAM17), and markers of blood-brain barrier integrity (ICAM-1 and VCAM-1) were measured in hippocampal tissue collected from adult and aged mice 6 h after administration of sgp130 (ICV) and LPS (i.p.). Table 2 shows the main effects of sgp130 and LPS on IL-6 and MHC-II mRNA. There was a sgp130 x LPS interaction in both IL-6 [F (2, 33) = 3.25, p < 0.04] and MHC-II [F (2, 33) = 5.57, p < 0.01] in aged but not in adult brain; whereby sgp130 decreased the basal and LPS-induced expression of IL-6 and MHC-II mRNA in the aged brain, but not in the adult brain. Treatment effects on the other genes that were assessed are also presented in Table 2. Of note, the age- related increase in ADAM17 mRNA was reduced by sgp130; suggesting a positive feedback loop between IL-6 trans-signaling and ADAM17in aged animals.
Table 2.
Hippocampal gene expression 6 h after sgp130 and LPS.
| Vehicle (ICV) | sgp130 (100 ng) (ICV) | p - value | ||||||
|---|---|---|---|---|---|---|---|---|
|
| ||||||||
| Age | Gene | Saline (i.p.) | LPS (10 μg) (i.p.) | Saline (i.p.) | LPS (10μg) (i.p.) | sgp130 | LPS | sgp130 × LPS |
| Adult | IL-1β | 0.99± 0.20 | 8.98± 2.54 | 1.25± 0.36 | 7.80± 1.48 | 0.873 | <0.001* | 0.536 |
| IL-6 | 1.34± 0.52 | 12.00± 4.61 | 0.864± 0.28 | 6.70± 2.29 | 0.096 | <0.001* | 0.180 | |
| MHC-II | 0.67± 0.26 | 0.79± 0.19 | 0.66± 0.22 | 0.83± 0.25 | 0.778 | 0.984 | 0.513 | |
| iNOS2 | 0.91± 0.16 | 2.71± 1.85 | 0.78± 0.28 | 2.73± 0.65 | 0.951 | 0.059 | 0.632 | |
| ADAM10 | 0.82± 0.20 | 1.38± 0.05 | 1.05± 0.15 | 1.17± 0.64 | 0.711 | 0.049* | 0.842 | |
| ADAM17 | 1.09± 0.17 | 0.98± 0.45 | 0.96± 0.23 | 0.88± 0.31 | 0.860 | 0.977 | 0.621 | |
| SOCS1 | 1.43± 0.30 | 1.34± 0.36 | 1.58± 0.35 | 1.32± 0.22 | 0.632 | 0.187 | 0.545 | |
| SOCS3 | 1.01± 0.10 | 7.69± 4.67 | 0.74± 0.14 | 5.39± 2.54 | 0.137 | <0.001* | 0.553 | |
| ICAM-I | 1.07± 0.25 | 3.76± 1.98 | 0.90± 0.10 | 2.22± 0.82 | 0.753 | 0.040* | 0.366 | |
| VCAM-I | 1.00± 0.07 | 2.08± 0.54 | 1.13± 0.28 | 1.40± 0.45 | 0.837 | 0.048* | 0.411 | |
|
|
||||||||
| Aged | IL-1β | 2.67± 0.99 | 16.53± 2.15 | 3.31± 1.17 | 10.97± 4.47 | 0.115 | <0.001* | 0.131 |
| IL-6 | 5.41± 2.23 | 22.75± 5.67 | 2.17± 0.85 | 11.10± 3.21 | 0.047* | <0.001* | 0.031* | |
| MHC-II | 1.97± 0.47 | 2.49± 1.12 | 0.88± 0.43 | 0.69± 0.21 | 0.051* | 0.009* | 0.039* | |
| iNOS2 | 0.98± 0.22 | 2.96± 1.26 | 0.78± 0.28 | 2.91± 1.84 | 0.971 | 0.045* | 0.732 | |
| ADAM10 | 1.06± 0.13 | 1.51± .18 | 1.07± 0.25 | 1.45± 0.27 | 0.643 | 0.057 | 0.115 | |
| ADAM17 | 2.17± 0.39 | 2.61± 0.82 | 1.30± 0.13 | 2.01± 0.36 | 0.043* | 0.321 | 0.431 | |
| SOCS1 | 0.82± 0.32 | 2.70± 1.93 | 1.17± 0.18 | 2.96± 0.54 | 0.718 | 0.074 | 0.333 | |
| SOCS3 | 1.23± 0.19 | 11.33± 2.55 | 1.09± 0.18 | 11.95± 2.20 | 0.340 | <0.001* | 0.513 | |
| ICAM-I | 4.34± 2.14 | 4.92± 2.64 | 1.81± 0.89 | 5.32± 1.35 | 0.110 | 0.101 | 0.215 | |
| VCAM-I | 1.86± 0.68 | 2.14± 0.80 | 1.29± 0.56 | 1.36± 0.42 | 0.221 | 0.445 | 0.655 | |
Fold Change
a significant difference per treatment group
Discussion
A recent study suggested that the magnitude and duration of IL-6 signaling are increased in the hippocampus of aged mice compared to adults after peripheral injection of LPS (Burton and Johnson, 2011). In the same study, sgp130 given ICV reduced IL-6 protein in the hippocampus and improved hippocampal-dependent learning and memory after i.p. injection of LPS (Burton and Johnson, 2011), suggesting a role for trans-signaling. The present study significantly extends this in several important ways. First, we are not aware of a previous study showing that more microglia from aged mice express IL-6R. Although the increased expression of IL-6R in conjunction with increased production of IL-6 helps explain the effects of aging on IL-6 signaling in the hippocampus, it also introduces the idea that microglia are an important source of sIL-6R in the aged brain. This is supported by our finding of higher ADAM17 mRNA in the hippocampus of aged mice. sIL-6R is necessary for trans-signaling, which is important in the CNS because neurons that express gp130 appear to express little IL- 6R (Burton et al., 2011). Thus, these data suggest that IL-6R shedding may be higher in the aged brain, which induces the pro-inflammatory IL-6 trans-signaling arm. Similar observations in models of colon cancer found an increase in ADAM17 and upregulated IL-6R on T-cells, connecting IL-6 trans-signaling to immune cell regulation and disease progression (Becker et al., 2004). It is well-documented that there are heightened levels of peripheral IL-6 in aged animals pre- and post LPS (Burton and Johnson, 2011; Godbout and Johnson, 2004). This inflammatory signal is sent to the brain through vagal, humoral, and diffusive pathways (Dantzer et al., 1999; Villeda et al., 2011). Once the signal is in the brain, the microenvironment becomes a factor and this study begins to elucidate a potential feed forward system in CNS-derived exaggeration of IL-6.
As a means to distinguish classical from trans-signaling in the brain, we injected sgp130 ICV, a molecule that competes with cell-bound gp130 for the IL-6-sIL-6R complex. Because sgp130 only binds the IL-6-sIL-6R complex, it does not interfere with classical signaling. Using this approach, LPS-induced sickness behavior was ameliorated in aged mice and the percentage of microglia expressing IL-6 was significantly reduced, as was the basal upregulated expression of several inflammatory genes in the hippocampus. Thus, another key finding in the present study was that IL-6 trans-signaling contributes to the induction of IL-6 expression in microglia and expression of other inflammatory molecules in the brain.
Another important finding was that whereas sgp130 reduced signs of illness in aged mice given LPS, it had little or no effect in similarly treated adults. Further, it should be noted that in aged mice sgp130 did not completely block the effects of LPS but rather returned them to what was seen in adults. Recent evidence suggests that the microenvironment of the normal aged brain is characterized by chronic low-level inflammation and increased microglia reactivity (Godbout and Johnson, 2009). In the present study, this was evident by an age-related increase in microglia that were positive for both MHC-II and IL-6. Furthermore, when mice were challenged with LPS, the increase in microglia that were positive for IL-6 was much greater in the aged, indicating enhanced reactivity. The exaggerated behavioral response seen in aged mice during infection is largely credited to the heightened reactivity of microglia. Thus, the present study suggests that an age-related increase in IL-6R and trans-signaling are major factors in eliciting behavioral pathology. The present study also suggests the “normal” behavioral response induced by peripheral LPS does not appear to involve trans-signaling because sgp130 did not inhibit sickness behavior in adults and only partially did so in the aged. This later point is important because the behavioral changes induced during infection are part of a well-organized response designed to help the infected host contend against the pathogen (Hart, 1988). The ability to constrain the response in aged animals without blocking it entirely is clinically relevant. This issue needs further investigation, however, because in another study using a different behavioral measurement (i.e., social behavior) sgp130 sped recovery after LPS injection (Burton et al., 2011).
Taken together, the results show that microglia are important regulators of the IL-6 trans-signaling response in the aged brain and that sgp130 exerts an anti-inflammatory effect by inhibiting the pro-inflammatory arm of IL-6 signaling. The fact that centrally administered sgp130 inhibited behavioral and neuroinflammatory responses to peripheral LPS in aged mice but not adults indicates trans-signaling is an important factor in the age-associated dysregulation of the neuroimmune network.
Research Highlight.
A cellular source of exaggerated central IL-6 is elucidated and the involvement of IL-6 trans-signaling on the exaggerated sickness response in the aged is identified.
Acknowledgments
The authors would like to thank Dr. Barbara Pilas (Flow cytometry core at the University of Illinois) for her technical expertise and assistance.
Funding
This research was supported by NIH grants; R01-AG16710 to R.W.J., DK064862 and NS058525 to G.G.F., and M.D.B. is supported by NIH diversity supplement SR01-AG16710.
List of abbreviations
- AP-1
Activator Protein-1
- APC
Allophycocyanin
- ADAM
A disintegrin and metalloprotease domain
- ANOVA
Analysis of variance
- BSA
Bovine Serum Albumin
- CNS
Central Nervous System
- CREB
cAMP response element-binding
- DMEM
Dulbecco’s Modified Eagle’s Medium
- EDTA
Ethylene diamine tetraacetic acid
- EGTA
Ethylene glycol tetraacetic acid
- FITC
Fluorescein Isothiocyanate
- FBS
Fetal Bovine Serum
- HBSS
Hank’s Balanced Salt Solution
- HEPES
4-(2-hydroxyethyl)-1-piperazineethanesulfonic acid
- HRP
Horseradish peroxidise
- ICV
Intracerebroventricular
- i.p
Intraperitoneal
- NFκB
Nuclear factor kappa B
- PE
R-Phycoerthrin
- SAS
Statistical Analysis Systems
- SEM
Standard Error of the Mean
- SDS
Sodium dodecyl sulphate
- Socs
Suppressor of cytokine signaling
Footnotes
Competing interests
The authors of this manuscript declare that they have no actual or potential competing interests.
Authors’ contributions
MDB was involved in research experimentation, completion of statistical analysis, and writing of the manuscript. JLR assisted with experimentation and data analysis. GGF and RWJ directed all aspects of this research project including experimental design, research experimentation, completion of statistical analysis, and writing of the manuscript.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Abraham J, Jang S, Godbout JP, Chen J, Kelley KW, Dantzer R, Johnson RW. Aging sensitizes mice to behavioral deficits induced by central HIV-1 gp120. Neurobiology of Aging. 2008;29:614–621. doi: 10.1016/j.neurobiolaging.2006.11.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Abraham J, Johnson RW. Central inhibition of interleukin-1 beta ameliorates sickness behavior in aged mice. Brain Behavior and Immunity. 2009;23:396–401. doi: 10.1016/j.bbi.2008.12.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Becker C, Fantini MC, Schramm C, Lehr HA, Wirtz S, Nikolaev A, Burg J, Strand S, Kiesslich R, Huber S, Ito H, Nishimoto N, Yoshizaki K, Kishimoto T, Galle PR, Blessing M, Rose-John S, Neurath MF. TGF-beta suppresses tumor progression in colon cancer by inhibition of IL-6 trans-signaling. Immunity. 2004;21:491–501. doi: 10.1016/j.immuni.2004.07.020. [DOI] [PubMed] [Google Scholar]
- Burton MD, Johnson RW. Interleukin-6 trans-signaling in the senescent mouse brain is involved in infection-related deficits in contextual fear conditioning. Brain Behavior and Immunity. 2011;26:738–738. doi: 10.1016/j.bbi.2011.10.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burton MD, Sparkman NL, Johnson RW. Inhibition of interleukin-6 trans-signaling in the brain facilitates recovery from lipopolysaccharide-induced sickness behavior. Journal of Neuroinflammation. 2011;8:54. doi: 10.1186/1742-2094-8-54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chalaris A, Gewiese J, Paliga K, Fleig L, Schneede A, Krieger K, Rose-John S, Scheller J. ADAM17-mediated shedding of the IL6R induces cleavage of the membrane stub by gamma-secretase. Biochimica et biophysica acta. 2010;1803:234–245. doi: 10.1016/j.bbamcr.2009.12.001. [DOI] [PubMed] [Google Scholar]
- Dantzer R, Aubert A, Bluthe RM, Gheusi G, Cremona S, Laye S, Konsman JP, Parnet P, Kelley KW. Mechanisms of the behavioural effects of cytokines. Cytokines, Stress, and Depression. 1999;461:83–105. doi: 10.1007/978-0-585-37970-8_6. [DOI] [PubMed] [Google Scholar]
- Ford AL, Goodsall AL, Hickey WF, Sedgwick JD. Normal adult ramified microglia separated from other central nervous system macrophages by flow cytometric sorting. Phenotypic differences defined and direct ex vivo antigen presentation to myelin basic protein-reactive CD4+ T cells compared. Journal of Immunology. 1995;154:4309–4321. [PubMed] [Google Scholar]
- Frank MG, Wieseler-Frank JL, Watkins LR, Maier SF. Rapid isolation of highly enriched and quiescent microglia from adult rat hippocampus: immunophenotypic and functional characteristics. Journal of Neuroscience Methods. 2006;151:121–130. doi: 10.1016/j.jneumeth.2005.06.026. [DOI] [PubMed] [Google Scholar]
- Godbout JP, Chen J, Abraham J, Richwine AF, Berg BM, Kelley KW, Johnson RW. Exaggerated neuroinflammation and sickness behavior in aged mice after activation of the peripheral innate immune system. FASEB Journal. 2005;19:1329. doi: 10.1096/fj.05-3776fje. [DOI] [PubMed] [Google Scholar]
- Godbout JP, Johnson RW. Interleukin-6 in the aging brain. Journal of Neuroimmunology. 2004;147:141–144. doi: 10.1016/j.jneuroim.2003.10.031. [DOI] [PubMed] [Google Scholar]
- Godbout JP, Johnson RW. Age and neuroinflammation: A lifetime of psychoneuroimmune consequences. Immunology and Allergy Clinics of North America. 2009;29:321. doi: 10.1016/j.iac.2009.02.007. [DOI] [PubMed] [Google Scholar]
- Hart BL. Biological basis of the behavior of sick animals. Neuroscience and biobehavioral reviews. 1988;12:123–137. doi: 10.1016/s0149-7634(88)80004-6. [DOI] [PubMed] [Google Scholar]
- Henry CJ, Huang Y, Wynne AM, Godbout JP. Peripheral lipopolysaccharide (LPS) challenge promotes microglial hyperactivity in aged mice that is associated with exaggerated induction of both pro-inflammatory IL-1 beta and anti-inflammatory IL-10 cytokines. Brain Behavior and Immunity. 2009;23:309–317. doi: 10.1016/j.bbi.2008.09.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jones SA, Richards PJ, Scheller J, Rose-John S. IL-6 transsignaling: The in vivo consequences. Journal of Interferon and Cytokine Research. 2005;25:241–253. doi: 10.1089/jir.2005.25.241. [DOI] [PubMed] [Google Scholar]
- Kishimoto T, Akira S, Taga T. IL-6 receptor and mechanism of signal transduction. International Journal of Immunopharmacology. 1992;14:431–438. doi: 10.1016/0192-0561(92)90173-i. [DOI] [PubMed] [Google Scholar]
- Krzyszton CP, Sparkman NL, Grant RW, Buchanan JB, Broussard SR, Woods J, Johnson RW. Exacerbated fatigue and motor deficits in interleukin-10-deficient mice after peripheral immune stimulation. American Journal of Physiology-Regulatory Integrative and Comparative Physiology. 2008;295:R1109–R1114. doi: 10.1152/ajpregu.90302.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matthews V, Schuster B, Schutze S, Bussmeyer I, Ludwig A, Hundhausen C, Sadowski T, Saftig P, Hartmann D, Kallen KJ, Rose-John S. Cellular cholesterol depletion triggers shedding of the human interleukin-6 receptor by ADAM10 and ADAM17 (TACE) Journal of Biological Chemistry. 2003;278:38829–38839. doi: 10.1074/jbc.M210584200. [DOI] [PubMed] [Google Scholar]
- Morley AA, Trainor KJ. Lack of an effect of vitamin E on lifespan of mice. Biogerontology. 2001;2:109–112. doi: 10.1023/a:1011589218219. [DOI] [PubMed] [Google Scholar]
- Nguyen K, D’Mello C, Le T, Urbanski S, Swain MG. Regulatory T cells suppress sickness behaviour development without altering liver injury in cholestatic mice. Journal of Hepatology. 2011 doi: 10.1016/j.jhep.2011.09.014. [DOI] [PubMed] [Google Scholar]
- Rose-John S. Interleukin-6 biology is coordinated by membrane bound and soluble receptors. Acta Biochimica Polonica. 2003;50:603–611. [PubMed] [Google Scholar]
- Rose-John S, Scheller J, Elson G, Jones SA. Interleukin-6 biology is coordinated by membrane-bound and soluble receptors: role in inflammation and cancer. Journal of Leukocyte Biology. 2006;80:227–236. doi: 10.1189/jlb.1105674. [DOI] [PubMed] [Google Scholar]
- Scheller J, Chalaris A, Schmidt-Arras D, Rose-John S. The pro- and anti-inflammatory properties of the cytokine interleukin-6. Biochim Biophys Acta. 2011;1813:878–888. doi: 10.1016/j.bbamcr.2011.01.034. [DOI] [PubMed] [Google Scholar]
- Schobitz B, Dekloet ER, Sutanto W, Holsboer F. Cellular localization of the interleukin-6 mRNA and interleukin-6 receptor mRNA in rat brain. European Journal of Neuroscience. 1993;5:1426–1435. doi: 10.1111/j.1460-9568.1993.tb00210.x. [DOI] [PubMed] [Google Scholar]
- Villeda SA, Luo J, Mosher KI, Zou B, Britschgi M, Bieri G, Stan TM, Fainberg N, Ding Z, Eggel A, Lucin KM, Czirr E, Park JS, Couillard-Despres S, Aigner L, Li G, Peskind ER, Kaye JA, Quinn JF, Galasko DR, Xie XS, Rando TA, Wyss-Coray T. The ageing systemic milieu negatively regulates neurogenesis and cognitive function. Nature. 2011;477:90–94. doi: 10.1038/nature10357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ye SM, Johnson RW. Increased interleukin-6 expression by microglia from brain of aged mice. Journal of Neuroimmunology. 1999a;93:139–148. doi: 10.1016/s0165-5728(98)00217-3. [DOI] [PubMed] [Google Scholar]
- Ye SM, Johnson RW. Increased interleukin-6 expression by microglia from brain of aged mice. Journal of Neuroimmunology. 1999b;93:139–148. doi: 10.1016/s0165-5728(98)00217-3. [DOI] [PubMed] [Google Scholar]
- Ye SM, Johnson RW. Regulation of interleukin-6 gene expression in brain of aged mice by nuclear factor kappa B. Journal of Neuroimmunology. 2001;117:87–96. doi: 10.1016/s0165-5728(01)00316-2. [DOI] [PubMed] [Google Scholar]