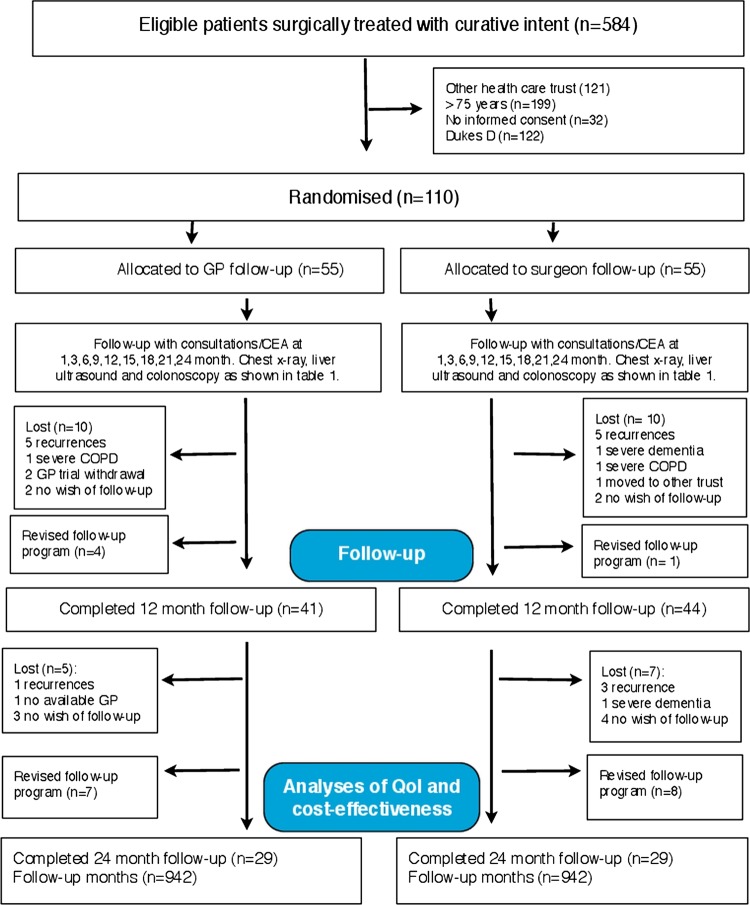
Figure 1.
Flow of participants. Patients were enrolled in the 2007 NGICG (Norwegian Gastrointestinal Cancer Group, table 1) follow-up programmes in both trial arms. The programmes are divided in 3-month cycles, that is, clinical examination at 1 (baseline), 3, 6, 9, 12, 15, 18, 21 and 24 months, carcinoembryonic antigen (CEA) measurement at 3-month intervals, chest x-ray and contrast-enhanced liver ultrasound every 6 months and colonoscopy once during 24 months (table 1).