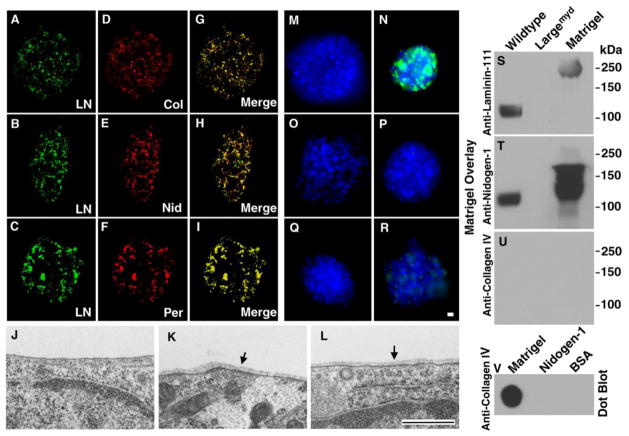
Figure 4. Co-localization of major BM components and formation of BM structure on neural stem cells after incubation with Matrigel.
(A–I) Wildtype neural spheres were incubated with Matrigel for 24 hrs. Bound proteins including Laminin-111, collagen IV, nidogen-1, and perlecan were visualized by double immunofluorescence staining. Note co-localization of laminin-111 with collagen IV (A, D, and G), nidogen-1 (B, E, and H), and perlecan (C, F, and I).
(J–L) Wildtype neural stem cells were incubated with Matrigel (K and L) or without Matrigel (J) followed by electron microscopy. Incubation with Matrigel produced BM structures (arrows in K and L). BM was not observed in the absence of Matrigel incubation (J).
(M–R) Incubation of wildtype neural sphere with (N) or without (M) laminin-111, with (P) or without (O) collagen IV, and with (R) or without (Q) nidogen-1. Significant binding was observed for laminin-111 while residual binding was found for nidogen-1. No binding was observed for collagen IV.
(S–U) Matrigel gel overlay assay. Glycoproteins isolated by WGA-agarose from wildtype and Largemyd mouse brains were separated on SDS-PAGE and blotted onto PVDF membrane. After incubation with Matrigel, the membrane was detected with antibodies against laminin-111 (S), nidogen-1 (T), and collagen IV (U). Matrigel lane and Matrigel dot-blotted onto PVDF membrane (V) served as positive controls for antibody detection. Although collagen IV antibody did not detect denatured proteins, it detected native collagen IV (V). While laminin-111 and nidogen-1 were detected at 125 kDa location (expected location of glycosylated α-DG) in the wildtype, they were not detected in Largemyd sample.
Abbreviations: KO, knockout; LN, laminin-111; Col, collagen IV; Nid, nidogen-1; Per, perlecan. Scale bar in R: 10 μm for A–I and M–R. Scale bar in L: 500 nm for J–L.