Abstract
Background
Therapeutic augmentation of fracture site angiogenesis with Deferoxamine (DFO) has proven to increase vascularity, callus size and mineralization in long-bone fracture models. We posit that the addition of DFO would enhance pathological fracture healing in the setting of radiotherapy in a model where non-unions are the most common outcome.
Methods
Sprague-Dawley rats (n = 35) were divided into 3 groups. Fracture (Fx), radiated fracture (XFx) and radiated fracture + DFO (XFxDFO). Groups XFx and XFxDFO received a human equivalent dose of radiotherapy (7 Gy/day × 5 days = 35 Gy) 2 weeks prior to mandibular osteotomy and external fixation. The XFxDFO group received injections of DFO into the fracture callus after surgery. Following a 40-day healing period, mandibles were dissected, clinically assessed for bony-union, imaged with Micro-CT, and tension tested to failure.
Results
Compared to radiated fractures, metrics of callus size, mineralization and strength in DFO treated mandibles were significantly increased. These metrics were restored to a level demonstrating no statistical difference from control fractures. In addition we observed an increased rate of achieving bony unions in the XFxDFO treated group when compared to XFx (67% vs. 20% respectively).
Conclusions
Our data demonstrate near total restoration of callus size, mineralization, and biomechanical strength, as well as a 3-fold increase in the rate of union with the use of DFO. Our results suggest that the administration of DFO may have the potential for clinical translation as a new treatment paradigm for radiation induced pathologic fractures.
Level of Evidence
Animal study, not gradable for level of evidence.
Introduction
Ensuring stability and adequate blood supply are clinical staples in fracture management. However, pathologic fractures in previously radiated bone pose a more complex clinical management obstacle due to limitations in fracture site vascularity and impediments to callus formation. In normal bone, early augmentations in angiogenesis are crucial to the development of an adequate callus capable of imparting structural integrity to the fracture site. Further, the stability imparted by that callus allows for the undisrupted interconnection of small maturing vessels, and the development and remodeling of the vascular microenvironment. These intimately connected variables function in a dynamic cycle that ultimately leads to the successful healing of bone across a fracture site.1–4 Callus size and strength are variables that are quantifiably diminished in previously radiated bone after fracture.5–7 Therefore, ensuring stability and adequate blood supply become priorities in the management of these pathologic fractures. Finding a means to prevent the impediments of radiotherapy on fracture vascularity and callus formation would thus have immense therapeutic potential.
Using fracture models, investigators have demonstrated an increase in blood flow that peaks at 7–14 days after fracture.8,9 Conceptually, therapeutic manipulation around this time-period may allow for early triggering and sustenance of angiogenic responses that lead to increased vascularity and accelerated fracture healing. Deferoxamine (DFO), an iron chelator, has a demonstrated capacity to increase angiogenesis via the hypoxia inducible factor (HIF 1-α) pathway. Investigators have shown quantifiable augmentations in fracture site vascularity with local injections of DFO into a fracture callus.10 Transient and localized increases in blood circulation secondary to fracture make the callus a potentially promising environment for therapeutic exploitation. Through a mechanism of iron chelation, DFO causes the accumulation of intracellular HIF 1-α. This elicits a transcriptional process that ultimately leads to the production of vascular endothelial growth factor (VEGF), as well as other downstream mediators of angiogenesis, resulting in the growth of new blood vessels.11 In addition, recent studies have demonstrated the osteogenic stimulus of VEGF to be comparable to bone morphogenetic protein 2 (BMP-2) in vivo.12,13
The aim of the present study is to test the hypothesis that locally injected Deferoxamine has the ability to improve callus size, mineralization and strength, as well as to increase the rate of union formation in a model of radiation impaired fracture healing.
Materials & Methods
All animals were subjected to radiotherapy and osteotomy surgery with select animals also receiving Deferoxamine (DFO) therapy. Animal experimentation was conducted in accordance with the guidelines published in the Guide for the Care and Use of Laboratory Animals: Eighth Edition. Protocols were approved by the University of Michigan’s Committee for the Utilization and Care of Animals (UCUCA) prior to implementation. The sample sizes needed to test our hypotheses correlating with our outcomes were determined prior to the study using power analysis with nQuery Advisor version 7.0 software and the assistance of the University of Michigan Center for Statistical Consultation and Research (CSCAR). Under the assumption that the data would be evaluated using a general linear model with associated analysis of variance with a desired power of 0.8 with a difference between groups of one standard deviation, we required at least five animals per group. Due to the addition of radiotherapy and biomechanical testing of weakened bone, we cautiously increased the sample sizes in the radiated groups.
Experimental Design
Twelve-week-old male Sprague Dawley rats weighing approximately 400g were acclimated for seven days in light and temperature controlled facilities and given food and water ad libitum. The rats were then randomly assigned to 3 groups: fracture (Fx, n=5), radiated fracture (XFx, n=15) and radiated fracture + DFO (XFxDFO, n=15). Radiotherapy was administered to the XFx and XFxDFO groups over a five day-period followed by a recuperation period of 14 days prior to surgery. During recovery, animals were acclimated to a soft chow high-calorie diet (Hills-Columbus Serum; Columbus, OH) to ensure adequate food intake and nutrition in the post-radiation and post-operative periods. Subsequently, all groups underwent mandibular osteotomy and external fixator placement. The XFxDFO group received localized injections of DFO (200 μmol/300 μL) into the fracture callus every other day for a total of 5 doses on POD 4–12. Following a 40-day healing period, mandibles were dissected en-bloc, clinically assessed for bony-union, imaged with Micro-CT (μCT), and tension tested to failure. ANOVA was used to analyze differences between metrics, and statistical significance was considered at p < 0.05.
Radiation
Induction of anesthesia was provided with an oxygen/isoflurane mixture. Left hemi-mandibles were radiated using a Philips RT250 orthovoltage unit (250 kV X-rays, 15 mA; Kimtron Medical, Woodbury, CT). Our region of interest (ROI) spanned a distance of 2 mm posterior to the third molar. This region correlated to the future site of osteotomy. Lead shielding ensured localized delivery and protection of surrounding tissues. A previously described Human Equivalent Dose of Radiation (HEDR) was utilized.14,15 A fractioned dose of 7 Gy per day was administered over 5 days for a total of 35 Gy. This is comparable to 70 Gy in human mandibular high-dose radiotherapy. Animals were allowed a 14-day recovery from radiation exposure prior to osteotomy surgery.
Surgery
After standard prepping and draping with the animal on its dorsum, a 2 cm midline incision was placed ventrally from the anterior submentum to the neck crease. Skin flaps were elevated and the anterior-lateral mandible was exposed. A horizontal through-and-through defect was drilled (1/32) to pass a 1.5″ #0–80 stainless steel threaded rod across, with both ends brought externally through the skin. This created the anterior portion of our modified external fixator. A 1cm incision was created over the masseter muscle to expose the angle of the mandible. A small defect was drilled 2mm anterior-superior to the mandibular angle, bilaterally, to secure a #0–80 threaded pin. This pin was secured with a titanium washer and nut then brought externally through the skin for the posterior fixator placement. The completed fixator was secured, then a vertical osteotomy was created using a10mm reciprocating saw blade directly behind the third molar on the left hemi mandible. The external fixator device was adjusted to insure reduction and hemostasis of the osteotomy edges. After reduction, the wounds were irrigated, hemostasis verified and the incisions were closed in layers. 17 Separation of our fracture gap post-operatively created an analyzable rectangular defect spanning 2mm behind the third molar. DFO injection was started on POD 4 to allow for the formation of an initial soft callus. The delivery of DFO into the fracture callus was done by grossly palpating the defect edges and injecting a small volume (300μl) into the callus. Methylene blue dye verification of injection was conducted to ensure delivery as previously described.20
Deferoxamine Injection
The XFxDFO group received a 200 μM DFO injection in 300 μL of normal saline (NS) directly into the fracture site every other day starting on post-operative day 4 and continuing through post-operative day 12. This dose was selected from a review of the literature concerning the use of DFO in animal models of bone healing and modified according to our experimental use of this therapy in the rat mandible.10,11,18–20 The timing of drug delivery was chosen to allow for the initial formation of a soft callus and to coincide with a previously reported time period for initiation of angiogenesis in murine fracture models.1,2,8
Micro-CT
A 40-day recovery period was allowed for fracture healing prior to outcome analysis. Mandibles were dissected en-bloc and assessed for bony union, which was clinically defined as an absence of movement across the fracture site on palpation after removal of the fixator device. μCT images were obtained using 80 kVp, 80 mA and 1100 ms exposures. Three hundred ninety two projections were taken at a 45-micron voxel size for bone analysis. General Electric’s Microview 2.2 software was used to generate our ROI, which includes all bone 2 mm posterior to 3rd molar with the incisor root excluded, and derive metrics of total volume (TV), bone volume (BV), bone mineral density (BMD), and tissue mineral content (TMC). Callus size was defined as TV, due to the fact that ROI only includes areas of callus formation.
Biomechanical Tension Testing
After imaging, mandibles were potted and loaded to failure in uniaxial monotonic tension at 0.5 mm/s using a servohydraulic 858 Minibiox II testing machine (MTS Systems Corporation; Eden Prairie, MN). Crosshead displacement was recorded by using an external linear variable differential transducer (LVDT; Lucas Schavitts, Hampton, VA), and load data were collected with a 100-lb load cell (Sensotec, Columbus, OH). Data were sampled at 200 Hz on a TestStar system (TestStar IIs System version 2.4; MTS Systems Corporation). Load-displacement curves were analyzed for ultimate load, yield load, intrinsic stiffness, yield energy, failure energy, and post-yield displacement using custom computational code (MATLAB 7.11; Mathworks Inc., Natick, MA).
Statistical Analysis
All statistical analysis was conducted using PSAW 19 software. All variables were compared using ANOVA with Tukey’s post hoc method. All data are presented as the means + SD. Statistical significance was assessed at p < 0.05.
Results
Biomechanical Tension Testing
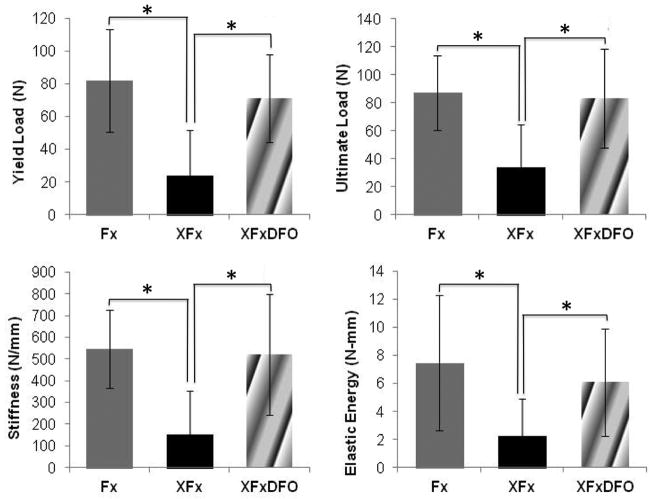
Two samples in the XFx group were not biomechanically tested due to technical failure. These animals were not replaced. Across biomechanical parameters assessed, there was a deleterious effect following radiation therapy and a restorative effect following DFO treatment. When comparing Fx to XFx rats, there were significant decreases in yield load (71%, p = 0.002), stiffness (72%, p = 0.014), ultimate load (61%, p = 0.03), and elastic energy (70%, p = 0.022). When comparing XFx to XFxDFO rats, there were significant increases in yield load (202%, p = 0.001), stiffness (243%, p = 0.001), ultimate load (147%, p = 0.003), and elastic energy (175%, p = 0.017). There were no significant differences between any of the aforementioned parameters between the Fx and XFxDFO groups, indicating that deferoxamine restores the irradiated mandibles back to the same biomechanical properties as the Fx group (Fig. 1).
Figure 1.
Yield load, ultimate load, elastic energy, and stiffness means and standard deviations demonstrating treatment effectiveness. * Denotes significance at p ≤ 0.05 between means.
Micro-CT
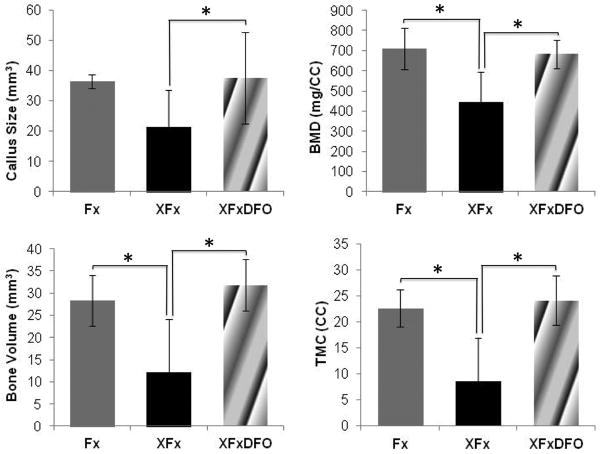
Callus volumetric and densitometric analyses demonstrated results congruent with biomechanical data. Across radiomorphometric parameters assessed, there was a deleterious effect following radiation therapy and a restorative effect following DFO treatment. When comparing Fx to XFx rats, there were significant decreases in bone mineral density (38%, p = 0.001), bone volume (57%, p = 0.004), tissue mineral content (62%, p = 0.001), and a trending decrease in callus size (42%, p = 0.058). The addition of DFO evidenced significant beneficial increases in bone mineral density (53%, p =0.001), bone volume (160%, p = 0.001), tissue mineral content (62%, p = 0.001), and callus size (77%, p = 0.001) when compared to the XFx group. Furthermore, these restored metrics were not statistically different from normal fracture repair indicating a restoration of fracture callus mineralization (Fig. 2).
Figure 2.
Callus size, BMD, BV, and TMC means and standard deviations demonstrating treatment effectiveness. * Denotes significance at p ≤ 0.05 between means.
Bony Union
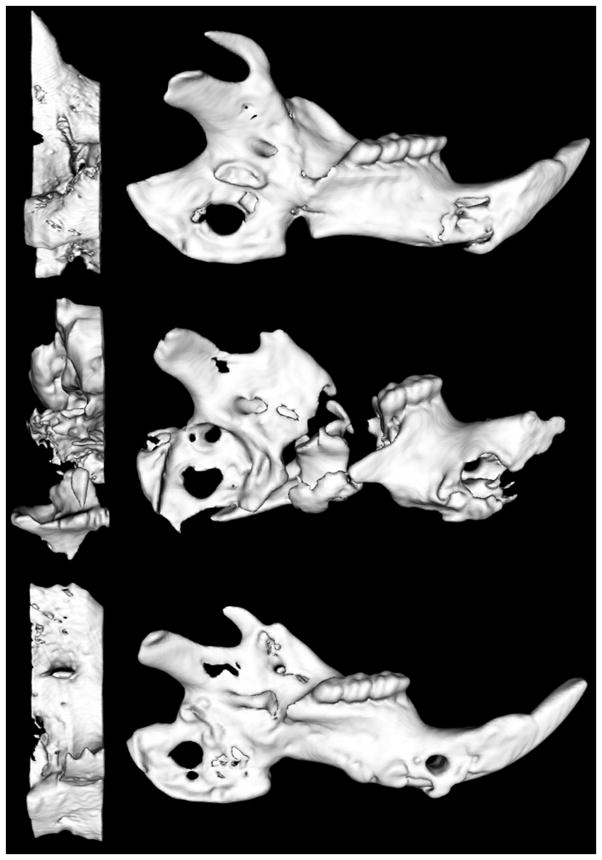
Control fractures (Fx) exhibited bony union in all cases (100%), radiated fractures (XFx) achieved a 20% union rate, and DFO treated fractures (XFxDFO) achieved an improved bony union rate of 67%. Further, unions in the DFO treated group exhibited enhanced bony bridging when clinically compared to radiated counterparts without treatment (Fig. 3). Quantitative metrics for μCT comparing only unions in the XFx group (n = 3) and the XFxDFO group (n = 10) showed a significant increase in BVF upon the addition of DFO by 26% (p = 0.009). In addition, we observed trending increases in TMC by 44% (p = 0.057) and in BMD by 23% (p = 0.058).
Figure 3.
Select μCT images depicting Fx, XFx and XFxDFO mandibles from top to bottom. (Left) Enlarged images demonstrate the corresponding ROI within each mandible. Notice the severe destruction and altered microstructure due to radiotherapy and the corresponding restitution of anatomy due to the addition of DFO therapy.
Discussion
Approximately 4–6% of all Head and Neck Cancer (HNC) patients who receive adjuvant radiotherapy will go on to develop bone related pathologies such as mandibular osteoradionecrosis, pathologic fractures and secondary non-unions.21 Currently, there are no proven therapeutic interventions that will aid in the prevention of non-unions after pathologic fractures occur. Present treatment strategies include debridement, rigid fixation, bone grafting and the controversial use of hyperbaric oxygen therapy (HBO).22,23 These approaches have not been uniformly efficacious; in fact, failure rates of many of these treatments reach as high as 40%.21 In cases where these treatments fail, surgeons have advocated the use of autologous free tissue transfer as a salvage procedure. Unfortunately even free tissue transfer is not consistently successful and is associated with significant cost and morbidity, particularly in the elderly and infirm.24–27 Thus, finding a remedy to prevent non-unions secondary to pathologic fractures in the setting of radiotherapy would have immense therapeutic ramifications.
Our initial goal was to establish a reproducible murine model of pathologic fracture healing that consistently produced quantifiable and clinically analogous detriments to fracture healing such as impaired mineralization, decreased mechanical strength and non-unions in the majority of cases. Ultimately, we aimed to restore these detriments by therapeutically augmenting vascularity during peak angiogenesis. This mechanism of restoration was selected based on the known impediments of radiation on the vascular microarchitecture and the subsequent effects that this limitation imposes on adequate callus formation and successful fracture healing.28–30
Previously, investigators have quantified the diminutions to callus size and strength secondary to radiation. Hayashi and Suit found that a significant proportion of radiated fracture calluses only achieved 30% of normal callus size via radiographic examination.7 Widmann et al found significant decreases in torque to failure and torsional stiffness with a 9 Gy dose given before fracture. Pelker et al demonstrated similar impediments to biomechanical strength with 11 Gy given before fracture.5,6 In order to expand upon previous investigations, we focused on the examination of the incidence and restoration of bony union. Thus, our model of radiation administration was more rigorous, and was based on a dose that was equivalent to what a patient undergoing high-dose radiotherapy would encounter.14,15 This fractionated Human Equivalent Dose of Radiation (HEDR) of 35Gy quantitatively exhibited significantly diminished parameters of biomechanical strength and callus mineralization, and ultimately produced non-unions in the majority of cases (80%).
Our consideration and selection of a therapeutic means to prevent non-unions was based on the specific criteria of vascular augmentation and potential clinical translation. Deferoxamine, Parathyroid Hormone, Mesenchymal Stem Cells, Bone Morphogenic Proteins and Sclerostin Antibodies are currently among the myriad of promising therapeutics demonstrating augmentations in callus size, bone quality and biomechanical strength in normal bone healing models.10,31–34 While the optimization of normal fracture healing bears import, the potential to utilize these agents in pathologic fractures would provide solutions to more complex dilemmas with limited management solutions. Our particular interest in DFO was based on a consideration of the effects of radiation on bone healing and remodeling. Radiated bone has a demonstrated diminished capacity to heal.35–38 These effects are largely associated with the impact of radiotherapy on the existing vascular microarchitecture and the natural physiologic processes of angiogenesis. Acutely, radiation directly impacts the existing microvasculature causing structural disruptions, functional compromise, decreased overall vascular density, and the obliteration of small blood vessels.28–30 Since radiotherapy is known to specifically target and diminish vascularity, we looked to use an angiogenic mechanism to bolster callus size, mineralization and strength, as well as to increase the rate of union formation in our model of pathologic fracture healing. Deferoxamine presented as a logical choice due to its acceptance in the clinical arena with a known and limited side effect profile for transfusion related iron-overload.39
Our results demonstrated the ability of DFO to remediate the effect of radiation on callus size, quality, and strength in our pathologic fracture model. A near 2-fold increase in callus TV was appreciated when comparing radiated fractures to those treated with DFO. Further, this callus size was comparable to normal fractures that had not been exposed to radiation therapy. Similar results were seen regarding BVF and TMC; metrics representing callus structure and mineralization, respectively. The addition of DFO restored μCT metrics of BVF and TMC to normal fracture levels by factors of approximately 1.5 and 3 respectively.
Previous investigation has shown that μCT outcome measures are strong predictors of mechanical properties.40 Our data corroborate these findings as our biomechanical data demonstrated results that were reflective of our μCT data. We observed a significant 3-fold increase in yield, a 3-fold increase in elastic energy, a 2.5-fold increase in ultimate load, and a 3-fold increase in stiffness when comparing radiated fractures to DFO treated counterparts. Particularly of interest are the metrics of yield point and ultimate load. While these variables are not clinically measurable, they represent valuable quantifiable metrics of non-recoverable deformation (plastic deformation) and the maximum load achieved prior to fracture, respectively. Clinically, radiologic changes in callus size and BMD may be indicators of when bone is too weak to withstand physiologically relevant loads. Closer follow-ups with CT scans may hold the potential as a predictor of impaired loading capacity.
To investigate the quality and strength of bony unions, we specifically compared unions in the radiated fracture group to unions in the treatment group. Although limitations in sample size precluded statistical significance in TMC and BMD, we observed a significant increase in BVF in the DFO treated group. This suggests that DFO not only allows the fractures to form unions with higher incidence, but that the unions formed with DFO are more mineralized and of higher quality those in the radiated fracture group. This was demonstrated clinically and radiographically as the 3 unions in the radiated fracture group had minimal bony bridging, while the 10 unions in the treatment group had more robust bony bridging reflective of our uCT data.
The importance of our findings must be weighed in the context of the limitations of the experimental model and in light of the proposed clinically relevant applications. It was our aim to investigate the effects of DFO in a scenario analogous to radiotherapy induced pathologic fractures. In order to establish a reproducible and controlled model, we utilized a surgically created osteotomy in lieu of the natural mechanisms leading to closed pathologic fractures. In reality, pathologic fractures are not surgically created, but spontaneously occur years after radiotherapy. Therefore, while our model is not clinically identical to the etiology of pathologic fractures, it does simulate the clinically analogous circumstances in which they are treated by open reduction. In both cases, bone healing is impaired by radiotherapy. It would stand to reason that if DFO can augment radiotherapy impaired fracture healing in our model, it may be able to provide a clinical solution to an otherwise devascularized area in order to avoid the devastating outcomes of non-unions. It is also important to note that despite a remarkable improvement in bony union from 20%–67% in treated mandibles, a restoration to 100% bony union was not achieved. Future studies will entail the consideration of synergistic combination therapies that may more predictably establish consistent unions despite radiotherapy.
Although our results are promising, it is important to note that we are proposing the use of an angiogenic agent after radiation into tissues previously surrounding a malignancy. This may raise concerns regarding the tumorigenic safety of this intended use. While there is no definitive study indicating that DFO is safe for use in oral cancer patients, there is research demonstrating DFO’s antitumorogenic effects via depletion of iron stores needed for DNA replication in multiple types of cancer.41–45 In addition, although radiotherapy has been shown to promote HIF 1-α activation, the consequences of such activation are complex and not fully understood. While the activation of the HIF 1-α pathway may protect tumor microvasculature from the cytotoxic effects of radiation, upregulation of HIF 1-α has been shown to promote apoptosis and decrease clonogenic survival of p-53+ tumor cells, thereby sensitizing them to be killed more efficiently by radiotherapy.46 In light of these findings, we advocate further investigation regarding this complex interplay involving this promising therapy and HNC.
Conclusion
We have demonstrated that DFO can restore quantifiable metrics of callus size, mineralization, and strength, which manifests in a substantive increase in the rate of successful bony unions. Ultimately, our results support the contention that for the purposes of optimizing fracture healing after radiotherapy, the augmentation of vascularity may be a promising mechanism of exploitation with real translational potential.
Acknowledgments
The authors thank Deniz Sarhaddi and Behod Poushanchi for laboratory assistance. Supported by grants from the National Institutes of Health (CA125187) to S.R.B and (T32-GM008616) to A.D.
Footnotes
Presented at the 57th Annual Meeting of the Plastic Surgery Research Council, June 2012, Ann Arbor, MI.
Financial Disclosure and Products
Funding supported by the following grants: “Optimization of Bone Regeneration in the Irradiated Mandible”, NIH-R01#CA 125187- 01, PI: Steven R. Buchman. “Training Grant in Trauma, Burn and Wound Healing Research”, NIH-T32-GM008616, for Alexis Donneys.
None of the authors has a financial interest in any of the products, devices, or drugs mentioned in this manuscript.
References
- 1.AI-Aql ZS, Alagl S, Graves DT, Gerstenfeld LC, Einhorn T. Molecular mechanisms controlling bone formation during fracture healing and distraction osteogenesis. J Dent Res. 2008;87(2):107–118. doi: 10.1177/154405910808700215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Glowacki J. Angiogenesis in fracture repair. Clin Orthop Relat Res. 1998;355(Suppl):S82–S89. doi: 10.1097/00003086-199810001-00010. [DOI] [PubMed] [Google Scholar]
- 3.Gerstenfeld LC, Alkhiary YM, Krall EA, Nicholls FH, Stapleton SN, Fitch JL. Three-dimensional reconstruction of fracture callus morphogenesis. J Histochem Cytochem. 2006;54:1215–1228. doi: 10.1369/jhc.6A6959.2006. [DOI] [PubMed] [Google Scholar]
- 4.Gerstenfeld LC, Cullinane DM, Barnes GL, Graves DT, Einhorn TA. Fracture healing as a post-natal developmental process: molecular, spatial, and temporal aspects of its regulation. J Cell Biochem. 2003;88:873–884. doi: 10.1002/jcb.10435. [DOI] [PubMed] [Google Scholar]
- 5.Pelker RR, Friedlaender GE. The Nicolas Andry Award-1995. Fracture healing radiation induced alterations. Clin Orthop Relat Res. 1997;341:267–282. [PubMed] [Google Scholar]
- 6.Widmann RF, Pelker RR, Friedlaender GE, Panjabi MM, Peschel RE. Effects of prefracture irradiation on the biomechanical parameters of fracture healing. J Orthop Res. 1993;11:422–428. doi: 10.1002/jor.1100110315. [DOI] [PubMed] [Google Scholar]
- 7.Hayashi S, Suit HD. Effect of fractionation of radiation dose on callus formation at site of fracture. Radiology. 1971;101:181–186. doi: 10.1148/101.1.181. [DOI] [PubMed] [Google Scholar]
- 8.Aronson J. Temporal and spatial increases in blood flow during distraction osteogenesis. Clin Orthop Relat Res. 1994;(301):124–31. [PubMed] [Google Scholar]
- 9.Williams E, Rand J, An K, Chao E, Kelly P. The early healing of tibial osteotomies stabilized by one-plane or two-plane external fixation. J Bone Joint Surg Am. 1987;69(3):355–365. [PubMed] [Google Scholar]
- 10.Shen X, Wan C, Ramaswamy G, et al. Prolyl hydroxylase inhibitors increase neoangiogenesis and callus formation following femur fracture in mice. J Orthop Res. 2009;27:1298–1305. doi: 10.1002/jor.20886. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Wan C, Gilbert SR, Cao X, et al. Activation of the hypoxia-inducible factor-1alpha pathway accelerates bone regeneration. Proc Natl Acad Sci USA. 2008;105:686–691. doi: 10.1073/pnas.0708474105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Behr B, Sorkin M, Lehnhardt M, Renda A, Longaker MT, Quarto N. A comparative analysis of the osteogenic effects of BMP-2, FGF-2, and VEGFA in a calvarial defect model. Tissue Eng Part A. 2012;18(9–10):1079–86. doi: 10.1089/ten.tea.2011.0537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Schmitt C, Lutz R, Doering H, Lell M, Ratky J, Schlegel KA. Bio-Oss® blocks combined with BMP-2 and VEGF for the regeneration of bony defects and vertical augmentation. Clin Oral Impl Res. 2011;00:1–11. doi: 10.1111/j.1600-0501.2011.02351.x. [DOI] [PubMed] [Google Scholar]
- 14.Tchanque-Fossuo CN, Monson LA, Farberg AS, Donneys A, Deshpande SS, Razdolsky ER, Halonen NR, Goldstein SA, Buchman SR. Dose-response effect of human equivalent radiation in the murine mandible. Plast Reconstr Surg. 2011;128(5):480e–487e. doi: 10.1097/PRS.0b013e31822b67ae. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Monson LA, Farberg AS, Jing XL, Buchman SR. Human equivalent radiation dose response in the rat mandible. Plast Reconstr Surg. 2009 Oct;124(4S):2. [Google Scholar]
- 16.Buchman SR, Ignelzi MA, Jr, Radu C, et al. A unique rodent model of distraction osteogenesis of the mandible. Ann Plast Surg. 2002;49:511–519. doi: 10.1097/00000637-200211000-00012. [DOI] [PubMed] [Google Scholar]
- 17.Tong L, Buchman SR, Ignelzi MA, Jr, Rhee S, Goldstein SA. Focal adhesion kinase expression during mandibular distraction osteogenesis: evidence for mechanotransduction. Plast Reconstr Surg. 2003 Jan;111(1):211–22. doi: 10.1097/01.PRS.0000033180.01581.9A. discussion 223–4. [DOI] [PubMed] [Google Scholar]
- 18.Street J, Bao M, deGuzman L, et al. Vascular endothelial growth factor stimulates bone repair by promoting angiogenesis and bone turnover. Proc Natl Acad Sci USA. 2002;99:9656–9661. doi: 10.1073/pnas.152324099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Farberg AS, Jing XL, Monson LA, Donneys A, Tchanque-Fossuo CN, Deshpande SS, Buchman SR. Deferoxamine reverses radiation induced hypovascularity during bone regeneration and repair in the murine mandible. Bone. 2012;50(5):1184–7. doi: 10.1016/j.bone.2012.01.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Donneys A, Farberg AS, Tchanque-Fossuo CN, Deshpande SS, Buchman SR. Deferoxamine enhances the vascular response of bone regeneration in mandibular distraction osteogenesis. Plast Reconstr Surg. 2012;129(4):850–856. doi: 10.1097/PRS.0b013e31824422f2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Coletti D, Ord RA. Treatment rationale for pathological fractures of the mandible: a series of 44 fractures. Int J Oral Maxillofac Surg. 2008;37(3):215–222. doi: 10.1016/j.ijom.2007.09.176. [DOI] [PubMed] [Google Scholar]
- 22.Marx RE, Johnson RP. Studies in the radiobiology of osteoradionecrosis and their clinical significance. Oral Surg Oral Med Oral Pathol. 1987;64(4):379–90. doi: 10.1016/0030-4220(87)90136-8. [DOI] [PubMed] [Google Scholar]
- 23.Marx RE. Osteoradionecrosis: a new concept of its pathophysiology. J Oral Maxillofac Surg. 1983;41(5):283–8. doi: 10.1016/0278-2391(83)90294-x. [DOI] [PubMed] [Google Scholar]
- 24.McCrory AL, Magnuson JS. Free tissue transfer versus pedicled flap in head and neck reconstruction. Laryngoscope. 2002;112(12):2161–2165. doi: 10.1097/00005537-200212000-00006. [DOI] [PubMed] [Google Scholar]
- 25.Nakamizo M, Yokoshima K, Yagi T. Use of free flaps for reconstruction in head and neck surgery: a retrospective study of 182 cases. Auris Nasus Larynx. 2004;31(3):269–273. doi: 10.1016/j.anl.2004.03.003. [DOI] [PubMed] [Google Scholar]
- 26.Ross DA, Hundal JS, Son YH, Ariyan S, Shin J, Lowlicht R, Sasaki CT. Microsurgical free flap reconstruction outcomes in head and neck cancer patients after surgical extirpation and intraoperative brachytherapy. Laryngoscope. 2004;114(7):1170–1176. doi: 10.1097/00005537-200407000-00007. [DOI] [PubMed] [Google Scholar]
- 27.Shaari CM, Buchbinder D, Costantino PD, Lawson W, Biller HF, Urken ML. Complications of microvascular head and neck surgery in the elderly. Arch Otolaryngol Head Neck Surg. 1998;124(4):407–411. doi: 10.1001/archotol.124.4.407. [DOI] [PubMed] [Google Scholar]
- 28.Cao X, Wu X, Frassica D, Yu B, Pang L, Xian L, Wan M, Lei W, Armour M, Tryggestad E, Wong J, Wen CY, Lu WW, Frassica FJ. Irradiation induces bone injury by damaging bone marrow microenvironment for stem cells. Proc Natl Acad Sci USA. 2011;108(4):1609–1614. doi: 10.1073/pnas.1015350108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Xie XT, et al. Experimental study of radiation effect on the mandibular microvasculature of the guinea pig. Chin J Dent Res. 1998;1(2):46–51. [PubMed] [Google Scholar]
- 30.Deshpande SS, Donneys A, Farberg AS, Tchanque-Fossuo CN, Zehtabzadeh AJ, Buchman SR. Quantification and characterization of radiation-induced changes to mandibular vascularity using micro-computed tomography. Plast Reconstr Surg. 2010;125(6):40. doi: 10.1097/SAP.0b013e318255a57d. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Holzer G, Majeska RJ, Lundy MW, Hartke JR, Einhorn TA. Parathyroid hormone enhances fracture healing. A preliminary report. Clin Orthop. 1999;366:258–63. doi: 10.1097/00003086-199909000-00033. [DOI] [PubMed] [Google Scholar]
- 32.Granero-Molto F, Weis JA, Miga MI, et al. Regenerative effects of transplanted mesenchymal stem cells in fracture healing. Stem Cells. 2009;27(8):1887–98. doi: 10.1002/stem.103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Schmidmaier G, Wildemann B, Cromme F, Kandziora F, Haas NP, Raschke M. Bone morphogenetic protein-2 coating of titanium implants increases biomechanical strength and accelerates bone remodeling in fracture treatment: A biomechanical and histological study in rats. Bone. 2002;30:816–822. doi: 10.1016/s8756-3282(02)00740-8. [DOI] [PubMed] [Google Scholar]
- 34.Agholme F, Li X, Isaksson H, Ke HZ, Aspenberg P. Sclerostin antibody treatment enhances metaphyseal bone healing in rats. J Bone Miner Res. 2010 doi: 10.1002/jbmr.135. Published online May 17, 2010. [DOI] [PubMed] [Google Scholar]
- 35.Brown RK, Pelker RR, Friedlaender GE, Peschel RE, Panjabi MM. Postfracture irradiation effects on the biomechanical and histologic parameters of fracture healing. J Orthop Res. 1991;9:876–882. doi: 10.1002/jor.1100090614. [DOI] [PubMed] [Google Scholar]
- 36.Jacobsson M, Albrektsson T, Turesson I. Dynamics of irradiation injury to bone tissue: A vital microscopic investigation. Acta Radiol Oncol. 1985;24:343–350. doi: 10.3109/02841868509136063. [DOI] [PubMed] [Google Scholar]
- 37.Jacobsson MG, Jonsson AK, Albrektsson TO, Turesson IE. Short and long-term effects of irradiation on bone regeneration. Plast Reconstr Surg. 1985;76:841–850. doi: 10.1097/00006534-198512000-00006. [DOI] [PubMed] [Google Scholar]
- 38.Sugimoto M, Takahashi S, Toguchida J, Kotoura Y, Shibamoto T, Yamamuro T. Changes in bone after high-dose irradiation: Biomechanics and histomorphology. J Bone Joint Surg (Br) 1991;73:492–497. doi: 10.1302/0301-620X.73B3.1670456. [DOI] [PubMed] [Google Scholar]
- 39.Brittenham GM. Iron-chelating therapy for transfusional iron overload. N Engl J Med. 2011;364:146–156. doi: 10.1056/NEJMct1004810. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Morgan EF, Mason ZD, Chien KB, et al. Micro-computed tomography assessment of fracture healing: relationships among callus structure, composition, and mechanical function. Bone. 2009;44:335–344. doi: 10.1016/j.bone.2008.10.039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Blatt J, Taylor SR, Stitely S. Mechanism of antineuroblastoma activity of deferoxamine in vitro. J Lab Clin Med. 1988;112:433. [PubMed] [Google Scholar]
- 42.Hann HWL, Stahlhut MW, Hann CL. Effect of iron and desferoxamine on cell growth and in vitro ferritin synthesis in human hepatoma cell lines. Hepatology. 1990;11:566–569. doi: 10.1002/hep.1840110407. [DOI] [PubMed] [Google Scholar]
- 43.Hann HWL, Stahlhut MW, Rubin R, Maddrey WC. Antitumor effect of deferoxamine in human hepatocellular carcinoma growing in athymic nude mice. Cancer. 1992;70:2051. doi: 10.1002/1097-0142(19921015)70:8<2051::aid-cncr2820700806>3.0.co;2-1. [DOI] [PubMed] [Google Scholar]
- 44.Donfrancesco A, Deb G, Dominici C, Pileggi D, Castello MA, Helson L. Effects of a single course of deferoxamine in neuroblastoma patients. Cancer Res. 1990;50:4929–4930. [PubMed] [Google Scholar]
- 45.Kulp KS, Vulliet PR. Mimosine blocks cell cycle progression by chelating iron in asynchronous human breast cancer cells. Toxicol Appl Pharmacol. 1996;139:356–364. doi: 10.1006/taap.1996.0176. [DOI] [PubMed] [Google Scholar]
- 46.Dewhirst MW, Cao Y, Li CY, Moeller B. Exploring the role of HIF-1 in early angiogenesis and response to radiotherapy. Radiother Oncol. 2007;83:249–255. doi: 10.1016/j.radonc.2007.05.016. [DOI] [PMC free article] [PubMed] [Google Scholar]