Abstract
Introduction
The fetus is dependent on the placenta for its supply of long chain polyunsaturated fatty acids (LCPUFA), which are essential in fetal growth and development. Previous work suggests that high maternal body mass index (BMI) inhibits fetal LCPUFA delivery and males have greater fatty acid requirements than females during development. We hypothesized that male placental fatty acid uptake would be more sensitive to maternal BMI compared to females.
Methods
Term placental samples were collected from healthy women receiving Cesarean section (n=38). Placental fatty acid transporter and binding protein gene expression and uptake of oleic acid (OA), arachidonic acid, (AA) and docosahexanoic acid (DHA) was measured. Two-way ANOVA was used to assess the effects of fetal sex and maternal overweight/obesity (BMI>26 kg/m2).
Results
Placental fatty acid uptake of OA was 43% lower in male offspring and 73% higher in female offspring of obese compared to normal BMI women (p<0.05). The interaction between fetal sex and maternal BMI had a significant effect on both OA (P=0.002) and AA uptake (P=0.01). DHA uptake was not affected by fetal sex or maternal obesity. Placental fatty acid transporter CD36 and binding protein FABP5 gene expression levels were lower in male offspring of obese mothers but were not affected by BMI among females.
Conclusion
Maternal obesity and fetal sex significantly affect the placental uptake of oleate and arachidonate. Placental fatty acid uptake in both male and female fetuses is sensitive to maternal BMI, but males may have inadequate acquisition of the unsaturated fatty acid OA, when exposed to maternal obesity.
Keywords: placenta, fatty acid uptake, fatty acid transporter, fetal sex, obesity
Introduction
Maternal or fetal deficiencies in long chain polyunsaturated fatty acids (LCPUFA) - particularly docosahexanoic acid (DHA) - during pregnancy result in decreased visual acuity, behavioral abnormalities and cognitive impairment in infants along with hypertension in adult offspring (1–3). The fetus depends on both a rich maternal fatty acid reservoir, and efficient placental transfer to meet its high demands for LCPUFA as its capacity for LCPUFA synthesis is not sufficient to meet its developmental requirements (4;5). Thus alterations in maternal supply or placental transport of LCPUFA affect fetal delivery and, potentially, developmental pathways.
Fatty acid levels in maternal plasma reflect her diet, metabolism and nutrient stores (6;7). In non-obese women, pre-pregnancy BMI is negatively associated with circulating maternal and fetal LCPUFA levels (7;8). Whether this relationship holds true for obese women (BMI > 30 kg/m2) is not known. However, it is known that the placentas of obese women have above normal levels of inflammation and oxidative stress (9;10), which may alter placental metabolism and transport pathways for fatty acids.
Considering the established effect of maternal BMI on other placental pathways (9;10), we suspect that obesity-induced suppression of fatty acid transport by the placenta would exacerbate the effects of low maternal LCPUFA supply and worsen fetal LCPUFA delivery. Consistent with this, a recent study showed that maternal obesity was associated with changes in placental fatty acid transport proteins and lower uptake of linoleic acid – the essential precursor of arachidonic acid (AA) - by the trophoblast (11). However, as the fetus has a limited ability to generate AA and DHA from their precursors (4;5) and depends largely on placental transport for its LCPUFA supply, the placental uptake of AA and DHA is of particular importance. Thus, there remains a significant gap in our understanding of maternal factors that regulate the uptake and transport of unsaturated fatty acids.
Tamimi et al, reported that women who are pregnant with boys consume more nutrients - 11% more animal-based lipids and 15% more vegetable-based lipids – as compared to those carrying girls (12). Furthermore, mouse studies have shown that inducing essential fatty acid deficiency during pregnancy, via a low-fat diet, led to a significant decrease in litter size due to increased male stillbirths, while the number of female pups was unchanged (13). Together these studies provide evidence that males either have higher nutritional requirements than females during development or that their transport system is inadequate when fatty acids are in short supply. If true, maternal body composition may have a powerful influence on lifelong health outcomes of offspring with boys being most sensitive.
We hypothesized that placental fatty acid uptake in both sexes would be suppressed with increasing maternal BMI but that males would be more severely affected. In order to test the hypothesis we measured placental uptake of monounsaturated oleic acid and the LCPUFA arachidonic acid and docosahexanoic acid in placentas of overweight and obese women (BMI >26 kg/m2) compared to placentas from women with a normal BMI (18.5–26 kg/m2).
Materials and Methods
Study subjects
Thirty-eight women at term (>37 weeks gestation) who were scheduled for Cesarean section were recruited at OHSU Labor & Delivery. Written, informed consent for tissue collection was obtained prior to surgery and approved by the Institutional Review Board. Exclusion criteria included multiple gestations; fetuses with chromosomal or structural anomalies including cardiac defects; diabetes (gestational or pre-existing); preeclampsia; maternal hypertension; and any other significant co-morbidity.
Maternal data obtained from the medical records included age, parity, race, gestational age at delivery, height and weight (pre-pregnancy, 1st/2nd/3rd trimester); neonatal data included birth weight, head circumference, crown-heel length and fetal sex. Maternal BMI was calculated from clinical measurements with the exception of pre-pregnancy weight which was self reported. Groups were stratified by early 1st trimester BMI because pre-pregnancy values were not available in every case. Pre-pregnancy and 1st trimester weight correlated well in the women where both were reported (R=0.99, P<0.0001, n=22). Placental tissue was collected at the time of Cesarean section for molecular analysis by sampling from four different cotyledons, removing maternal decidua and immediately snap freezing in liquid nitrogen. Tissue was stored at −80°C prior to analysis.
Tissue collection and fatty acid uptake experiments
In a subset of women (n=13), fresh placental tissue was collected at time of Cesarean section for explant studies following the method of Siman et al. with some modifications (14). Immediately following delivery, four 1cm × 1cm pieces of placenta were collected from different cotyledons avoiding areas that were necrosed or calcified, followed by removal of the chorionic plate and decidua. Tissue was placed in Dulbecco’s phosphate buffered saline (with magnesium and calcium, HyClone®, Logan, UT) warmed to 37°C. Small (<2mm 3) fragments of villous tissue were dissected. Placental fragments (four per well, each from a different cotyledon) were transferred to Netwell culture dishes containing 1.5 ml of uptake buffer (Hank’s balanced salt solution [HBSS] supplemented with 10mM HEPES, pH 7.4) and incubated at 37°C for 20 minutes for equilibration.
Fatty acid uptake experiments followed the method of Xu et al., modified for explants (15). Albumin-bound fatty acids (ratio of 1:1 fatty acid:BSA) were prepared by adding radio-labeled 1-14C-oleic acid, 1-14C-arachidonic acid or 1-14C–DHA (Moravek Biochemicals, Brea, CA) and corresponding non-labeled fatty acids (oleic acid [OA] (Acros Organics, NJ, USA), arachidonic acid [AA; Sigma, St. Louis, MO] and docosahexanoic acid [DHA; Cayman Chem, Ann Arbor, MI] for a total fatty acid concentration of 200 μM in uptake buffer. Explants were incubated with the radio-labeled fatty acids for multiple time points (0, 15, 30, 45, 60 min) at 37°C. To stop the reaction, ice cold “stop buffer” was added to each well (uptake buffer with 0.1% BSA and 200μM phloretin (MP Biomedicals, Solon, OH). Explants were washed three times with cold stop buffer and once with cold PBS. Explants were solubilized in 500 μl of Biosol™ (National Diagnostics, Atlanta, GA) at 50°C for 2 hours. A 100 μL aliquot was counted on a Beckman LS3801 Liquid Scintillation Counter (Beckman Coulter, Brea, CA). An additional aliquot was used to determine total protein concentration and counts/nmol fatty acid was determined by counting 100 μl of the uptake solution added to each well for each fatty acid (total counts). Uptake was calculated for each well by dividing counts/well by counts/nmol and normalized by protein concentration. Placental fatty acid uptake (nmol/mg/min) was calculated by plotting the slope of the line of uptake over time of incubation (0–60 min). This relationship was determined to be linear within the time period analyzed using a runs test. There was no degradation of the syncytiotrophoblast membrane during this time period based on histological analysis using hematoxylin and eosin staining of sections of explants (data not shown).
Placental gene expression analysis by qPCR
Total RNA was isolated following homogenization of ~50 mg of tissue (representing four different cotyledons) in TriReagent (Sigma) following the manufacturer’s protocol. RNA was not treated with DNase as we have previously found this can compromise the integrity of the RNA. RNA integrity was assessed for each sample by visualizing ribosomal RNA via gel electrophoresis. We have previously assessed RNA integrity via Agilent BioAnalysis (details) and found sample RIN 8.4–9.3 for placental RNA extracted using this methodology. Reverse transcription of 2 μg RNA to cDNA was performed using MultiScribe Reverse Transcriptase (50U/μl) with random primers following manufacturer’s guidelines and cycling conditions (Applied Biosystems High-capacity cDNA RT kit (4368814), Carlsbad, CA). cDNA was stored at −20°C. GAPDH, a gene that was not altered in the placenta by BMI, was used as a reference gene. Gene-specific primers were designed for fatty acid transport proteins (FATP)-4, (FATP)-2; fatty acid translocase (CD36); fatty acid binding proteins (FABP)-3, (FABP)-4, (FABP)-5, (FABPpm) and lipoprotein lipase (LPL) using primer design software (Clone Manager Professional Suite, v.8). Primers were designed to span multiple intron-exon boundaries. Primer sequences and gene information are shown in Table 1. qPCR was performed as described previously (16). PCR amplicons were detected by fluorescent detection of SYBR Green following manufacturer’s instructions (Applied Biosystems Power SYBR® Green Master Mix (4368708)) and cycling conditions, using the Stratagene Mx3005P Thermocycler (Agilent Technologies, Santa Clara, CA). Cycling conditions were the same for all primer pairs: 95°C 10 min (for enzyme activation) followed by 40 cycles of 95°C 20s, 55°C for 30s, 72 °C 30s. For each primer pair, a standard curve, no template controls and unknowns were run in triplicate. Following cycling, the melt curve of the resulting amplicon was analyzed to ensure that a single product was detected for every replicate (95°C 1 mi n; 55°C 30s ramping to 95°C 30s, and collecting fluorescence data at every degree increase). Efficiency of each primer set was calculated using the slope of the respective standard curves with manufacturer’s software (MxPro v4.10; Stratagene) (see Table 1). The quantification cycle (Cq) was calculated for each replicate using MxPro software for detecting the amplification-based threshold. Replicates were not used if >1 SD of the Cq. NTCs were not detectable ≤ 40 cycles. Comparative quantification corrected for the efficiency of the respective standard curve was used to generate values for each replicate based on the Cq using MxPro software. The same sample was used as the “calibrator” in all assays. Values were expressed as a ratio of the gene of interest:reference gene in each sample.
Table 1.
Real time PCR primers
| Gene | Gene ID | Sense (5′→3′) | Antisense (5′→3′) | Product Size (bp) | Efficiency (%) |
|---|---|---|---|---|---|
| FABPpm | NM_002080.2 | TTGCTGCTGCCATTCTGAAC | AGGCTTTAGCCCTGTGAAAC | 188 | 85 |
| CD36 | NM_001001548 | AAACCTCCTTGGCCTGATAG | GAATTGGCCACCCAGAAACC | 173 | 120 |
| FABP-3 | NM_004102.3 | GACACTGGATGGAGGGAAAC | GTAGCCGATTGGCAGAGTAG | 191 | 99 |
| FABP-4 | NM_001442.2 | TACTGGGCCAGGAATTTGAC | GAAGTGACGCCTTTCATGAC | 178 | 120 |
| FABP-5 | NM_001444.2 | TTTCTTGTACCCTGGGAGAG | TCCGAGTACAGGTGACATTG | 195 | 115 |
| FATP-2 | NM_001159629.1 | TTCTATGCTGCCACTGAAGG | GGAACTCTGACGCAATATCC | 173 | 112 |
| FATP-4 | NM_005094.3 | GCTGCCCTGGTGTACTATGG | GGAGGCTGAAGAACTTCTTCC | 153 | 95 |
| LPL | NM_000237.2 | GGGCATGTTGACATTTACCC | CAAAGGCTTCCTTGGAACTG | 211 | 97 |
| GADPH | NM_002046.4 | AGGTGGTCTCCTCTGACTTC | TACTCCTTGGAGGCCATGTG | 169 | 95 |
Statistical analysis
Two-way repeated measures analysis of variance followed by Bonferroni post-test was used to examine differences between groups in time-dependent experiments (GraphPad Prism 4.03). Two-way ANOVA was used to examine the effect of offspring gender and maternal obesity on placental fatty acid uptake and gene expression. Bonferroni post-test was used to assess differences in rates of uptake between normal and obese groups for both sexes. Data are presented as mean ± SEM unless noted otherwise. P value <0.05 was considered statistically significant.
Results
Male (n=19) and female (n=19) neonates were stratified by maternal 1st trimester BMI into normal weight (BMI<26 kg/m2) and overweight/obese (BMI>26 kg/m2) groups (Table 2). Maternal obesity was associated with a higher birth weight and ponderal index in male and female offspring compared to offspring of lean women. Obese women carrying male offspring had lower gestational weight gain compared to lean women. Male neonates had higher birth weights (P=0.002), crown-heel lengths (P=0.004), head circumferences (P=0.002) and gestational ages (P=0.035) compared to females regardless of maternal BMI. Maternal age and nulliparous status were not different between groups. Maternal ethnicity information was available from the medical charts for 28 women. Thirteen women were Hispanic. The proportion of Hispanic women in each group was as follows: lean women with male offspring – 20%; lean women with female offspring – 11%; obese women with male offspring – 33%; obese women with female offspring – 70%. The proportion of Hispanic women was highest among obese women with female offspring, although the difference was not statistically significant between groups (Chi-squared: P=0.25; Fisher’s exact: P=0.51).
Table 2.
Maternal and Neonatal characteristics.
| MALES | FEMALES | ||||||
|---|---|---|---|---|---|---|---|
| Normal (n=10) | Obese (n=9) | Normal (n=9) | Obese (n=10) | Gender Effect (P value) | Obesity Effect (P value) | BMI* Gender Interaction (P value) | |
| Maternal age at delivery (y) | 32 ± 7 | 29 ± 5 | 34 ± 4 | 31 ± 5 | 0.263 | 0.097 | 1.000 |
| >35 years | 4 (40%) | 2 (22%) | 6 (67%) | 4 (40%) | - | - | 1.000# |
| Gestational age at delivery (weeks) | 39.3 ± 0.7 | 39.2 ± 0.3 | 38.7 ± 0.7 | 38.9 ± 0.7 | 0.035 | 0.808 | 0.468 |
| Nulliparous | 1 | 0 | 2 | 0 | - | - | - |
| 1st trimester BMI (kg/m2) | 21.2 ± 1.9 | 32.6 ± 4.2* | 21.2 ± 3.5 | 36.2 ± 6.2* | 0.202 | <0.0001 | 0.202 |
| Gestational weight gain (kg) | 15.8 ± 3.0 | 7.0 ± 7.6* | 12.6 ± 4.7 | 11.8 ± 5.6 | 0.736 | 0.056 | 0.105 |
| Birth weight (g) | 3.63 ± 0.28 | 3.95 ± 0.46 | 3.17 ± 0.38 | 3.53 ± 0.48 | 0.002 | 0.014 | 0.880 |
| Birth length (cm) | 52 ± 1 | 52 ± 1 | 50 ± 2 | 50 ± 3 | 0.004 | 1.000 | 1.000 |
| Birth HC (cm) | 36 ± 2 | 36 ± 1 | 35 ± 1 | 34 ± 1 | 0.002 | 0.279 | 0.279 |
| Ponderal index (g/cm3*100) | 2.5 ± 0.2 | 2.9 ± 0.2* | 2.6 ± 0.2 | 2.9 ± 0.3* | 0.519 | <0.0001 | 0.519 |
BMI=body mass index; HC=head circumference. Data are mean ± SD. All data analyzed by two-way analysis of variance, unless otherwise specified.
P<0.05 vs leaner group for respective sex by Bonferroni post-test.
P value calculated by Fisher’s exact test for differences between groups.
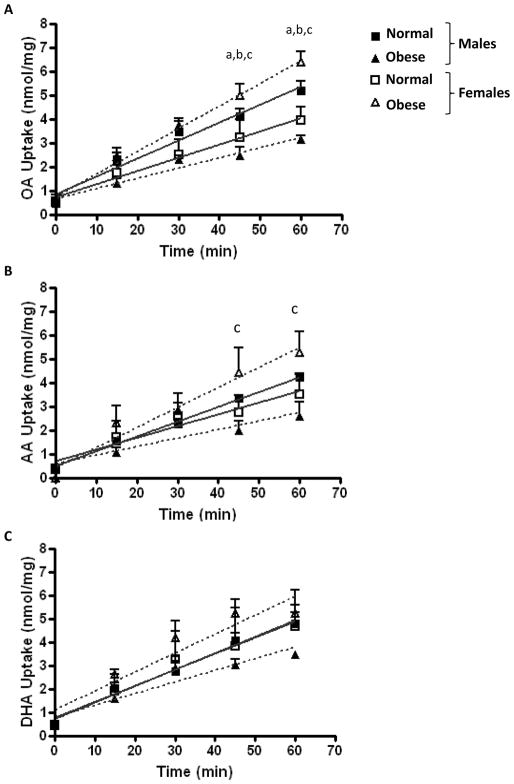
Time-dependent fatty acid uptake results are presented in Figure 1. Oleic acid (OA) uptake was lower in male offspring of obese women as compared to lean (mean ± SD: 2.45 ± 0.67 vs 4.09 ± 0.63 nmol/mg/45 min and 3.15 ± 0.28 vs 5.19 ± 0.78 nmol/mg/60 min; P<0.05), and was lower in male offspring than in female offspring of obese women (2.45 ± 0.67 vs 4.97 ± 0.84 nmol/mg/45 min and 3.15 ± 0.28 vs 6.38 ± 0.75 nmol/mg/60 min; P<0.001) at both 45 and 60 min (Fig. 1A). Placental uptake of both OA and arachidonic acid (AA) was higher in female offspring of obese women as compared to lean (OA: 4.97 ± 0.84 vs 3.21 ± 1.28 nmol/mg/45 min and 6.38 ± 0.75 vs 3.93 ± 0.97 nmol/mg/60 min; AA: 4.41 ± 1.89 vs 2.74 ± 1.23 nmol/mg/45 min and 5.26 ± 1.50 vs 3.51 ± 1.44 nmol/mg/60 min P<0.05) at 45 and 60 min (Fig. 1A & 1B). Time-dependent DHA uptake did not differ between groups (Fig. 1C).
Figure 1. Time-dependent placental fatty acid uptake in male and female offspring of normal and overweight/obese women.
Oleic acid (A) and arachidonic acid (B) placental uptake differed between groups at 45 and 60 min. Placental DHA uptake did not differ between groups at any time point studied (C). N=3–4 in each group. aP<0.05 male offspring of obese (▲) vs normal (■) women; bP<0.05 female offspring of obese (△) vs normal (□) women; c P<0.05 male vs female offspring of obese women by Bonferroni’s post-test.
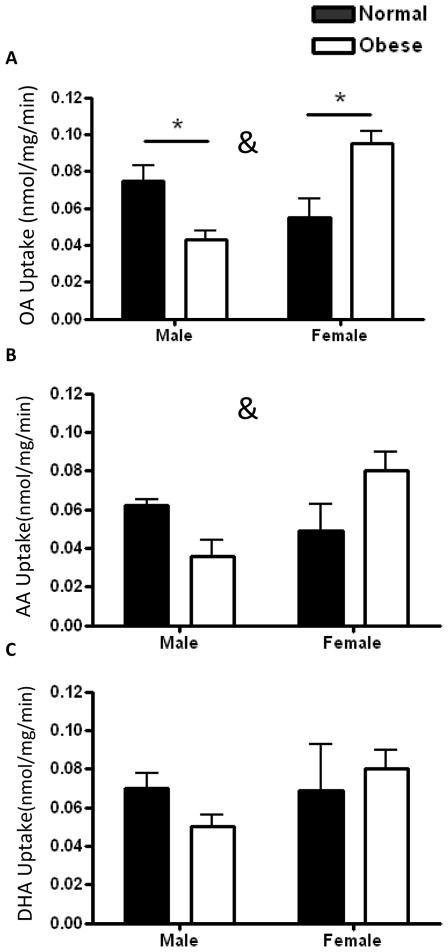
The rate (nmol/mg/min) of uptake of OA (Figure 2A) in placentas from males born to obese women was 43% lower than in male placentas from normal BMI women (0.043 ± 0.003 vs 0.075 ± 0.01; P=0.03). In contrast, placental oleic acid uptake was 73% higher in female offspring of obese compared to leaner women (0.095 ± 0.010 vs 0.055 ± 0.013; P=0.02) (Fig 2A). Placenta uptake of AA followed the same trends as oleic acid, but was not statistically significant (0.036 ± 0.021 vs 0.062 ± 0.006; P=0.08 in male placentas between maternal BMI groups; 0.084 ± 0.021 vs 0.049 ± 0.017; P=0.06 in female placentas) (Fig 2B). There was a significant interaction between offspring gender and maternal obesity for placental OA (P=0.001) and AA (P=0.01) uptake. Placental DHA uptake was not affected by gender or obesity (Fig. 2C). The overall rates of uptake did not differ between the different fatty acids (OA: 0.068 ± 0.022; AA: 0.058 ± 0.023; DHA: 0.068 ± 0.023) nor did the rates within lean/obese male/female groups.
Figure 2. Placental fatty acid uptake rates in male and female offspring of normal and overweight/obese women.
Oleic acid (OA) placental uptake decreased in males and increased in female offspring of obese mothers (A). Arachidonic acid uptake was not statistically significantly different between groups. Placental DHA uptake was unaffected by maternal obesity in either sex (C). N=3–4 in each group. * P<0.05 vs. normal placenta by Bonferroni’s post-test. &P<0.05 for interaction between obesity and offspring gender.
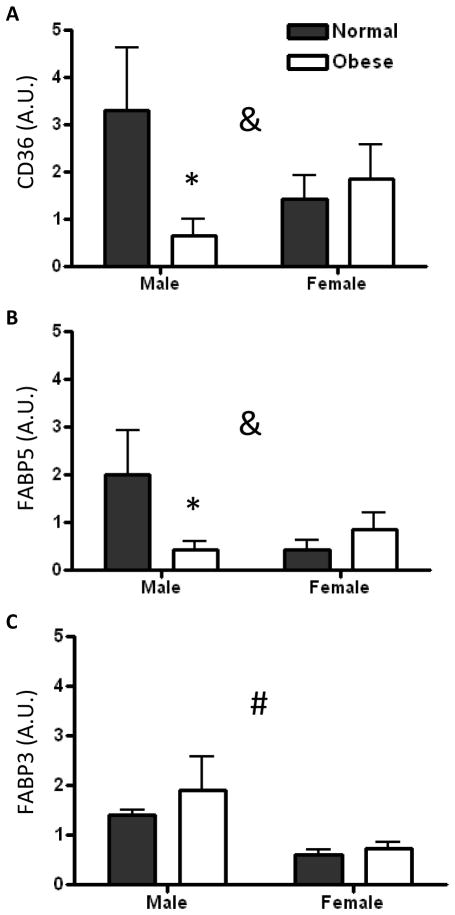
We measured mRNA levels of fatty acid transporters and binding proteins to determine the effect of maternal obesity on their expression levels. Placental CD36 (Figure 3A) and FABP5 (Fig. 3B) were decreased among males in the obese group (p<0.05). Among females, gene expression levels were unchanged with maternal obesity. There was a significant interaction between offspring gender and maternal obesity for placental CD36 (P=0.026) and FABP5 (P=0.009) expression. Placental FABP3 expression was higher in male offspring than female offspring (P=0.001) regardless of maternal BMI (Fig. 3C). A trend for an interaction between offspring gender and maternal obesity was seen for placental FATP2 expression, but this was not statistically significant (P=0.07). There were no significant detectable differences in placental gene expression of LPL, FATP4, FABP4, and FABPpm between groups.
Figure 3. Placental fatty acid transporter and binding protein gene expression in male and female offspring of normal and overweight/obese women.
Placental CD36 (A) and FABP5 (B) expression decreased in male offspring of obese mothers but was unaffected in female offspring. Placental FABP3 expression was higher in males than females (C). Units are arbitrary. N=6–7 in each group * P<0.05 vs. normal placenta by Bonferroni’s post-test. &P<0.05 for interaction between obesity and offspring gender. #P<0.05 for effect of offspring gender.
Discussion
The primary finding of this study is that placental villous tissue takes up unsaturated fatty acids at different rates according to fetal sex and maternal body phenotype. We found that placental uptake of oleic acid was suppressed among male offspring of obese women, but increased in female offspring. Placental uptake of the omega-3 LCPUFA DHA was not affected by maternal obesity in either males or females while uptake of the omega-6 LCPUFA AA was higher in female than male offspring of obese women. The decreased fatty acid uptake in male offspring of obese mothers was associated with a decrease in fatty acid binding protein-5 and fatty acid translocase (FAT/CD36), suggesting that these molecules are involved in the regulation of placental oleic acid uptake. These findings support our hypothesis that maternal obesity alters placental fatty acid transport systems in a sex-dependent manner.
Our study did not measure transplacental transport. Nevertheless, placental uptake is a necessary step in the materno-fetal transport process. The considerable sex-specific effect of maternal obesity on placental oleic acid uptake suggests that the delivery of more fatty acids than LCPUFA is closely regulated by the placenta. Oleic acid is the primary monounsaturated fatty acid in the fetal circulation and though its role in fetal development has not been as well studied as the LCPUFA, it has well-established cardiovascular effects with important anti-inflammatory and anti-oxidant activity in adults (17). Altered availability of oleate to the developing fetus may alter the composition of cellular membranes, affecting their ability to combat oxidants and alter their permeability. Our data suggest that male offspring of obese women may be at particular risk for these outcomes due to their suppressed placental uptake of oleate.
The finding that the interaction between maternal obesity and fetal sex is responsible for nearly 50% of the variability in the uptake of the LCPUFA arachidonic acid (AA) is also noteworthy because of the well established role of LCPUFA in neurological and cardiovascular development. In the US, 1 in 5 women of reproductive age are obese (18) and adverse pregnancy and neonatal complications associated with obesity during pregnancy are well known (recently reviewed by 19). Neonates born to obese mothers have increased risk for macrosomia, growth restriction, stillbirth/mortality, and adult-onset obesity, diabetes, and cardiovascular diseases (19–23). It is possible that some of these poor long-term outcomes in offspring of obese women may be related to alterations in LCPUFA or monounsaturated fatty acid supply during development (2;3), and that risk for these diseases depends upon the sex of the offspring. Perhaps not surprisingly, the placental uptake of DHA – the most preferentially transported fatty acid – was relatively protected from the effects of maternal obesity in both sexes. This is likely a well-protected mechanism not easily influenced in the uncomplicated pregnancies we studied.
The high rates of placental OA and AA uptake in female offspring of obese women suggests that female fetuses may be better able to compensate for variations in fatty acid delivery across the range of maternal BMI. This is consistent with a conservative growth strategy where females maintain a greater placental reserve than males and are better equipped for variations in maternal diet (24–26). Conversely, the male growth strategy is characterized by the development of a highly efficient placenta, which sacrifices its placental reserve in order to promote rapid fetal growth (25). This dangerous growth strategy for male fetuses imparts vulnerability during shortages in nutrient supply. Indeed, males have been shown to do more poorly in high-risk pregnancies than female offspring (27–29). Our data support the idea of a perilous male-specific growth strategy whereby placentas are less able to maintain unsaturated fatty acid uptake in association with maternal obesity.
The uptake and transplacental trafficking of fatty acids from maternal blood into the fetal circulation involves several families of proteins. Fatty acid transporter proteins 2, 4, and 6 (FATPs), fatty acid translocase (CD36), plasma membrane fatty acid binding protein (FABPpm) and the cytosolic binding proteins (FABP3, 4, 5) are highly expressed in trophoblasts and modulate fatty acid uptake in the human placenta (11;30–32). AA and DHA appear to be preferentially transferred across the placenta and maintain relatively higher concentrations in cord plasma than other fatty acids (33–35). It is not well-understood what transporters are responsible for this preferential uptake and delivery of the LCPUFA, though CD36 and FABPpm are believed to be non-specific for fatty acid type, while other transporters may have high specificity for LCPUFA (30;31). These proteins bind and transport non-esterified fatty acids that are either found in the circulation bound to albumin, or released from maternal triglycerides and phospholipids via the action of lipases (i.e. LPL) (30). We used albumin-bound fatty acids in our uptake experiments which would not take into account any alterations in lipase activity that may be present between lean and obese women. Consistent with previous findings, we did not observe any differences in LPL mRNA expression between groups, however this does not preclude alterations in LPL activity (11).
In our study, uptake of DHA was maintained in placentas of obese women while OA and AA were not. These results support the notion that there are fatty acid-specific mechanisms underlying placental uptake, allowing for differential regulation of individual fatty acid handling by the placenta in a sex-specific manner. Similar to DHA uptake, placental FATP4 expression was not affected by maternal obesity. Placental FATP4 mRNA expression has previously been demonstrated to be positively correlated with relative DHA levels in the cord blood (31), and is a potential mechanism underlying the maintenance of DHA uptake. It has recently been suggested that the acyl-CoA synthetase activity of FATP4 is responsible for regulating fatty acid uptake in adipocytes (36), though this has not been thoroughly investigated in the trophoblast.
Consistent with previous findings (11;37), our results show that alterations in fatty acid transporter expression are associated with changes in fatty acid uptake. We observed that the expression of the placental fatty acid transporter CD36 and the cytosolic binding protein FABP5 mirror placental OA uptake in males. CD36 expression has previously been shown to be necessary for OA uptake in cultured rat aortic smooth muscle cells (38). FABP5 may assist uptake by binding to the newly transported fatty acid once it enters the trophoblast, preventing it from leaving the cell, and trafficking lipids throughout the trophoblast (30;39). In females, the gene expression of fatty acid transporters was un-affected by maternal obesity suggesting that fatty acid uptake rates in female placentas may be regulated by post-transcriptional mechanisms, such as transporter protein trafficking to the membrane as seen in other tissues (40). These findings differ slightly from those of Dube et al. who found that maternal obesity was associated with higher placental CD36 levels (11). The differences between the findings of the two studies may be due to differences in study populations and/or that we analyzed male and female neonates separately, though we did not show differences between normal and obese groups if males and females were pooled (data not shown). Our finding of lower placental FABP5 expression in offspring of obese women was consistent with previous results (11). We also observed a strong effect of fetal gender on the expression of placental FABP3 regardless of maternal obesity, which may contribute to differential handling of fatty acids between male and female placentas at baseline. Though we did not find an overall difference in fatty acid uptake between male and female placentas, we cannot discount the possibility of sex-differences in placental lipid metabolism related to FABP3 expression, ultimately affecting fatty acid delivery to the fetus (37).
Limitations of our study should be considered. Despite a relatively small sample size, we saw highly significant effects of maternal obesity and fetal sex on placental fatty acid uptake and transporter expression. Secondly, fatty acid levels in the maternal and umbilical cord blood were not obtained. We do not expect to observe a direct correlation between placental fatty acid uptake and corresponding neonatal fatty acid levels because of the complexities surrounding placental lipid metabolism and transport (37). As we did not collect maternal blood, we are currently unable to ascertain what maternal metabolic factors related to obesity (i.e. insulin resistance, leptin, inflammation) may be associated with placental fatty acid uptake. We are currently designing follow-up studies to investigate these associations.
In summary, the study reported here shows that maternal early pregnancy BMI interacts with fetal sex to alter placental uptake of the LCPUFA AA and monounsaturated oleate along with fatty acid transporter expression. Consistent with our hypothesis, maternal obesity is associated with lower oleic acid uptake, but only in placentas of male offspring. These data suggest that male fetuses born to obese mothers pay a higher developmental price than do females with compromised uptake of a fatty acid important in the adult for normal cellular function. These findings point to the presence of important maternal signals, determined by maternal phenotype, that regulate the formation of the placenta and its function differently depending upon the sex of the offspring.
Acknowledgments
We thank Robert Webber for his excellent technical assistance. This research was supported by the National Institute of Child Health & Development (1K99HD062841). This work was carried out in the laboratory of Kent L. Thornburg.
Footnotes
Disclosures: Nothing to disclose
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Contributor Information
Elizabeth Brass, Email: brass@ohsu.edu.
Emily Hanson, Email: hanson@ohsu.edu.
Reference List
- 1.Armitage JA, Pearce AD, Sinclair AJ, Vingrys AJ, Weisinger RS, Weisinger HS. Increased blood pressure later in life may be associated with perinatal n-3 fatty acid deficiency. Lipids. 2003;38(4):459–464. doi: 10.1007/s11745-003-1084-y. [DOI] [PubMed] [Google Scholar]
- 2.Weisinger HS, Armitage JA, Sinclair AJ, Vingrys AJ, Burns PL, Weisinger RS. Perinatal omega-3 fatty acid deficiency affects blood pressure later in life. Nat Med. 2001;7(3):258–259. doi: 10.1038/85354. [DOI] [PubMed] [Google Scholar]
- 3.Connor WE, Neuringer M. The effects of n-3 fatty acid deficiency and repletion upon the fatty acid composition and function of the brain and retina. Prog Clin Biol Res. 1988;282:275–294. [PubMed] [Google Scholar]
- 4.Chambaz J, Ravel D, Manier MC, Pepin D, Mulliez N, Bereziat G. Essential fatty acids interconversion in the human fetal liver. Biol Neonate. 1985;47(3):136–140. doi: 10.1159/000242104. [DOI] [PubMed] [Google Scholar]
- 5.Szitanyi P, Koletzko B, Mydlilova A, Demmelmair H. Metabolism of 13C-labeled linoleic acid in newborn infants during the first week of life. Pediatr Res. 1999;45(5 Pt 1):669–673. doi: 10.1203/00006450-199905010-00010. [DOI] [PubMed] [Google Scholar]
- 6.Herrera E. Implications of dietary fatty acids during pregnancy on placental, fetal and postnatal development--a review. Placenta. 2002;23 (Suppl A):S9–19. doi: 10.1053/plac.2002.0771. [DOI] [PubMed] [Google Scholar]
- 7.Wijendran V, Bendel RB, Couch SC, Philipson EH, Thomsen K, Zhang X, et al. Maternal plasma phospholipid polyunsaturated fatty acids in pregnancy with and without gestational diabetes mellitus: relations with maternal factors. Am J Clin Nutr. 1999;70(1):53–61. doi: 10.1093/ajcn/70.1.53. [DOI] [PubMed] [Google Scholar]
- 8.Wijendran V, Bendel RB, Couch SC, Philipson EH, Cheruku S, Lammi-Keefe CJ. Fetal erythrocyte phospholipid polyunsaturated fatty acids are altered in pregnancy complicated with gestational diabetes mellitus. Lipids. 2000;35(8):927–931. doi: 10.1007/s11745-000-0602-2. [DOI] [PubMed] [Google Scholar]
- 9.Roberts KA, Riley SC, Reynolds RM, Barr S, Evans M, Statham A, et al. Placental structure and inflammation in pregnancies associated with obesity. Placenta. 2011;32(3):247–254. doi: 10.1016/j.placenta.2010.12.023. [DOI] [PubMed] [Google Scholar]
- 10.Roberts VH, Smith J, McLea SA, Heizer AB, Richardson JL, Myatt L. Effect of increasing maternal body mass index on oxidative and nitrative stress in the human placenta. Placenta. 2009;30(2):169–175. doi: 10.1016/j.placenta.2008.11.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Dube E, Gravel A, Martin C, Desparois G, Moussa I, Ethier-Chiasson M, et al. Modulation of Fatty Acid Transport and Metabolism by Obesity in the Human Full-Term Placenta. Biol Reprod. 2012 doi: 10.1095/biolreprod.111.098095. [DOI] [PubMed] [Google Scholar]
- 12.Tamimi RM, Lagiou P, Mucci LA, Hsieh CC, Adami HO, Trichopoulos D. Average energy intake among pregnant women carrying a boy compared with a girl. BMJ. 2003;326(7401):1245–1246. doi: 10.1136/bmj.326.7401.1245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Rivers JP, Crawford MA. Maternal nutrition and the sex ratio at birth. Nature. 1974;252(5481):297–298. doi: 10.1038/252297a0. [DOI] [PubMed] [Google Scholar]
- 14.Siman CM, Sibley CP, Jones CJ, Turner MA, Greenwood SL. The functional regeneration of syncytiotrophoblast in cultured explants of term placenta. Am J Physiol Regul Integr Comp Physiol. 2001;280(4):R1116–R1122. doi: 10.1152/ajpregu.2001.280.4.R1116. [DOI] [PubMed] [Google Scholar]
- 15.Xu Y, Cook TJ, Knipp GT. Methods for investigating placental fatty acid transport. Methods Mol Med. 2006;122:265–284. doi: 10.1385/1-59259-989-3:265. [DOI] [PubMed] [Google Scholar]
- 16.O’Tierney PF, Lewis RM, McWeeney SK, Hanson MA, Inskip HM, Morgan TK, et al. Immune response gene profiles in the term placenta depend upon maternal muscle mass. Reprod Sci. 2012;19(10):1041–1056. doi: 10.1177/1933719112440051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Schwingshackl L, Strasser B, Hoffmann G. Effects of monounsaturated fatty acids on cardiovascular risk factors: a systematic review and meta-analysis. Ann Nutr Metab. 2011;59(2–4):176–186. doi: 10.1159/000334071. [DOI] [PubMed] [Google Scholar]
- 18.Chu SY, Kim SY, Bish CL. Prepregnancy Obesity Prevalence in the United States, 2004–2005. Matern Child Health J. 2008 doi: 10.1007/s10995-008-0388-3. [DOI] [PubMed] [Google Scholar]
- 19.Aviram A, Hod M, Yogev Y. Maternal obesity: implications for pregnancy outcome and long-term risks-a link to maternal nutrition. Int J Gynaecol Obstet. 2011;115 (Suppl 1):S6–10. doi: 10.1016/S0020-7292(11)60004-0. [DOI] [PubMed] [Google Scholar]
- 20.Boney CM, Verma A, Tucker R, Vohr BR. Metabolic syndrome in childhood: association with birth weight, maternal obesity, and gestational diabetes mellitus. Pediatrics. 2005;115(3):e290–e296. doi: 10.1542/peds.2004-1808. [DOI] [PubMed] [Google Scholar]
- 21.Catalano PM, Presley L, Minium J, Hauguel-de Mouzon S. Fetuses of obese mothers develop insulin resistance in utero. Diabetes Care. 2009;32(6):1076–1080. doi: 10.2337/dc08-2077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Mills JL, Troendle J, Conley MR, Carter T, Druschel CM. Maternal obesity and congenital heart defects: a population-based study. Am J Clin Nutr. 2010;91(6):1543–1549. doi: 10.3945/ajcn.2009.28865. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Nohr EA, Bech BH, Davies MJ, Frydenberg M, Henriksen TB, Olsen J. Prepregnancy obesity and fetal death: a study within the Danish National Birth Cohort. Obstet Gynecol. 2005;106(2):250–259. doi: 10.1097/01.AOG.0000172422.81496.57. [DOI] [PubMed] [Google Scholar]
- 24.Mao J, Zhang X, Sieli PT, Falduto MT, Torres KE, Rosenfeld CS. Contrasting effects of different maternal diets on sexually dimorphic gene expression in the murine placenta. Proc Natl Acad Sci U S A. 2010;107(12):5557–5562. doi: 10.1073/pnas.1000440107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Eriksson JG, Kajantie E, Osmond C, Thornburg K, Barker DJ. Boys live dangerously in the womb. Am J Hum Biol. 2009 doi: 10.1002/ajhb.20995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Crawford MA, Doyle W, Meadows N. Gender differences at birth and differences in fetal growth. Hum Reprod. 1987;2(6):517–520. doi: 10.1093/oxfordjournals.humrep.a136581. [DOI] [PubMed] [Google Scholar]
- 27.Naeye RL, Demers LM. Differing effects of fetal sex on pregnancy and its outcome. Am J Med Genet Suppl. 1987;3:67–74. doi: 10.1002/ajmg.1320280509. [DOI] [PubMed] [Google Scholar]
- 28.Di Renzo GC, Rosati A, Sarti RD, Cruciani L, Cutuli AM. Does fetal sex affect pregnancy outcome? Gend Med. 2007;4(1):19–30. doi: 10.1016/s1550-8579(07)80004-0. [DOI] [PubMed] [Google Scholar]
- 29.Sheiner E, Levy A, Katz M, Hershkovitz R, Leron E, Mazor M. Gender does matter in perinatal medicine. Fetal Diagn Ther. 2004;19(4):366–369. doi: 10.1159/000077967. [DOI] [PubMed] [Google Scholar]
- 30.Duttaroy AK. Transport of fatty acids across the human placenta: a review. Prog Lipid Res. 2009;48(1):52–61. doi: 10.1016/j.plipres.2008.11.001. [DOI] [PubMed] [Google Scholar]
- 31.Larque E, Demmelmair H, Klingler M, De Jonge S, Bondy B, Koletzko B. Expression pattern of fatty acid transport protein-1 (FATP-1), FATP-4 and heart-fatty acid binding protein (H-FABP) genes in human term placenta. Early Hum Dev. 2006;82(10):697–701. doi: 10.1016/j.earlhumdev.2006.02.001. [DOI] [PubMed] [Google Scholar]
- 32.Mishima T, Miner JH, Morizane M, Stahl A, Sadovsky Y. The expression and function of fatty acid transport protein-2 and -4 in the murine placenta. PLoS One. 2011;6(10):e25865. doi: 10.1371/journal.pone.0025865. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Larque E, Demmelmair H, Berger B, Hasbargen U, Koletzko B. In vivo investigation of the placental transfer of (13)C-labeled fatty acids in humans. J Lipid Res. 2003;44(1):49–55. doi: 10.1194/jlr.m200067-jlr200. [DOI] [PubMed] [Google Scholar]
- 34.Campbell FM, Gordon MJ, Dutta-Roy AK. Preferential uptake of long chain polyunsaturated fatty acids by isolated human placental membranes. Mol Cell Biochem. 1996;155(1):77–83. doi: 10.1007/BF00714336. [DOI] [PubMed] [Google Scholar]
- 35.Gil-Sanchez A, Larque E, Demmelmair H, Acien MI, Faber FL, Parrilla JJ, et al. Maternal-fetal in vivo transfer of [13C]docosahexaenoic and other fatty acids across the human placenta 12 h after maternal oral intake. Am J Clin Nutr. 2010;92(1):115–122. doi: 10.3945/ajcn.2010.29589. [DOI] [PubMed] [Google Scholar]
- 36.Zhan T, Poppelreuther M, Ehehalt R, Fullekrug J. Overexpressed FATP1, ACSVL4/FATP4 and ACSL1 increase the cellular fatty acid uptake of 3T3-L1 adipocytes but are localized on intracellular membranes. PLoS One. 2012;7(9):e45087. doi: 10.1371/journal.pone.0045087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Xu Y, Agrawal S, Cook TJ, Knipp GT. Maternal di-(2-ethylhexyl)-phthalate exposure influences essential fatty acid homeostasis in rat placenta. Placenta. 2008;29(11):962–969. doi: 10.1016/j.placenta.2008.08.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Ma S, Yang D, Li D, Tang B, Yang Y. Oleic acid induces smooth muscle foam cell formation and enhances atherosclerotic lesion development via CD36. Lipids Health Dis. 2011;10:53. doi: 10.1186/1476-511X-10-53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Scifres CM, Chen B, Nelson DM, Sadovsky Y. Fatty acid binding protein 4 regulates intracellular lipid accumulation in human trophoblasts. J Clin Endocrinol Metab. 2011;96(7):E1083–E1091. doi: 10.1210/jc.2010-2084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Bonen A, Luiken JJ, Arumugam Y, Glatz JF, Tandon NN. Acute regulation of fatty acid uptake involves the cellular redistribution of fatty acid translocase. J Biol Chem. 2000;275(19):14501–14508. doi: 10.1074/jbc.275.19.14501. [DOI] [PubMed] [Google Scholar]