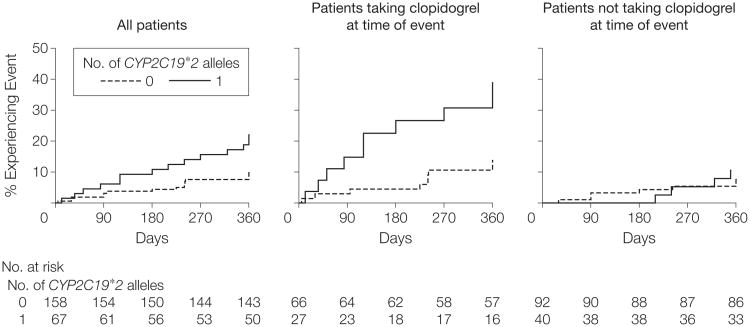
Figure 4. Event-Free Survival Over 1 Year of Follow-up in Sinai Hospital of Baltimore Patients Treated With Clopidogrel Following Percutaneous Coronary Intervention, Stratified by CYP2C19*2 Genotype.
Postdischarge ischemic events were defined as myocardial infarction (the occurrence of ischemic symptoms and a troponin I value greater than upper limits of normal), ischemic stroke, stent thrombosis (definite stent thrombosis according to the Academic Research Consortium criteria29), unplanned target vessel revascularization (revascularization of vessel treated at time of study enrollment), unplanned nontarget vessel revascularization (revascularization of a vessel different from that treated at time of study enrollment), hospitalization for coronary ischemia without revascularization (hospitalization for chest pain with evidence of ischemia on electrocardiogram and no evidence of myocardial infarction as measured by troponin I value), and death secondary to any cardiovascular cause. Patients were further stratified into those who were taking clopidogrel when the event occurred or at 1 year of follow-up and those who were not. All analyses adjusted for age, sex, and race. For all patients, hazard ratio (HR)=2.42 (95% confidence interval [CI], 1.18-4.99; P=.02); for patients taking clopidogrel at time of event, HR=3.40 (95% CI, 1.36-8.46; P=.004); for patients not taking clopidogrel at time of event, HR=1.39 (95% CI, 0.39-4.88; P=.60).