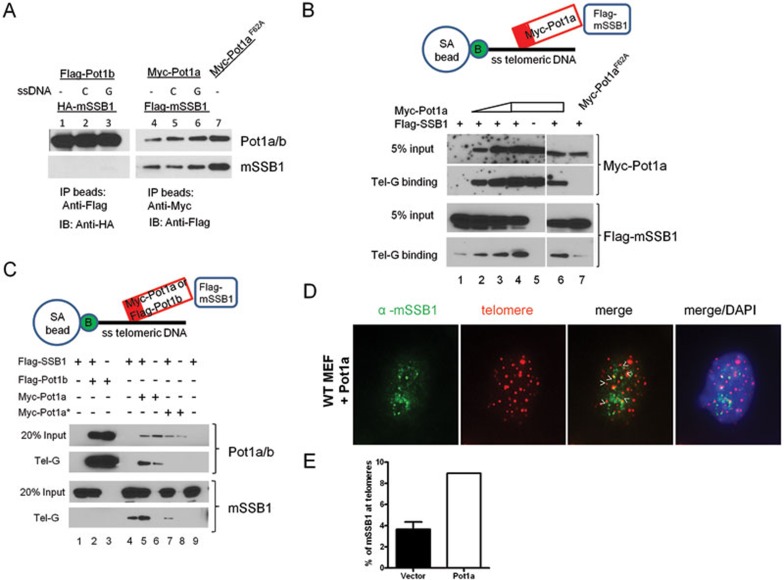
Figure 6.
mSSB1 interacts with Pot1a on telomeric ssDNA. (A) Detection of interaction between Pot1a, Pot1b and mSSB1 by coimmunoprecipitation. Epitope-tagged proteins were expressed in 293T cells and whole-cell lysates (WCL) were probed for proein-protein interactions. After binding of Flag-Pot1b, Myc-Pot1a or Myc-Pot1aF62A to anti-Flag or anti-Myc beads, the beads were incubated first with ssDNA (Tel-C (CCCTAA)6 or Tel-G (TTAGGG)6) and then with HA-SSB1 or Flag-SSB1. Anti-Flag or anti-Myc antibodies were used to detect bound Pot1b and Pot1a; anti-HA and anti-Flag antibodies were used to detect coimmunoprecipitated SSB1. (B) The schematic of the experiment is illustrated. Streptavidin (SA) beads coated with biotinylated (B) telomeric ssDNA (Tel-G: (TTAGGG)6) were incubated with 293T WCLs containing different ratios of Myc-Pot1a and Flag-SSB1. Proteins bound to the biotinylated ssDNA were detected by western blot analysis with anti-Myc (to detect Pot1a) or anti-Flag (to detect mSSB1) antibodies. Pot1aF62A was used to determine whether the Pot1a ssDNA-binding ability was required for mSSB1 interaction with biotinylated ssDNA. The OB-fold of Pot1a or Pot1b is illustrated as a red box. (C) Similar to B, a biotinylated telomeric ssDNA was used to show that mSSB1 interacts preferentially with Pot1a but not Pot1b. Pot1a* denotes the use of Pot1aF62A as a substitute for WT Pot1a. (D) Overexpression of Pot1a in WT MEFs resulted in increased colocalization of endogenous mSSB1 with telomeres. Telomere localization was assayed with PNA-probe (Cy3-OO-(CCCTAA)4 (red)). (E) Quantification of endogenous SSB1 colocalized to telomere in D. Cells with more than three colocalized SSB1 to telomere foci were scored positive.