Summary
Background and objective
The effects of recombinant human growth hormone on renal osteodystrophy are unknown; thus, the effects of growth hormone (GH) on bone histomorphometry were assessed in pediatric patients with ESRD.
Design, setting, participants, & measurements
Thirty-three patients who underwent bone biopsy between July 1994 and May 1999 were randomly assigned to therapy with or without GH. Patients were stratified by bone formation rate; all patients with high bone turnover received intraperitoneal calcitriol. Serum biochemical values were obtained monthly, and bone biopsy was repeated after 8 months.
Results
Median patient age was 11.7 years (interquartile range [IQR], 7.6, 14.1 years); 45% of patients were male, and 52% were prepubertal. Median dialysis duration was 0.4 (IQR, 0.3, 0.8) year. Bone formation rate per bone surface increased from 15.0 (9.6, 21.8) to 154.6 (23.7, 174.3) μm2/μm3 per year (P=0.05) in patients with low bone turnover treated with GH, decreased from 103.3 (57.0, 173.4) to 60.3 (20.3, 13.7) μm2/μm3 per year in patients with high bone turnover receiving standard therapy (P=0.03), and was unchanged in the other two groups. Bone formation rates were higher with GH, irrespective of underlying bone histologic features (P=0.05). Parathyroid hormone did not differ between groups. GH therapy resulted in greater increases in height SD scores (estimated mean difference in change ± SD, 0.324±0.076; P<0.001), irrespective of underlying bone histologic features.
Conclusions
GH therapy improves height in pediatric dialysis patients, irrespective of underlying bone histologic features. Bone formation rates are higher in GH recipients, and GH therapy alters the relationship between circulating parathyroid hormone values and bone turnover.
Introduction
Growth failure, resulting in part from resistance to the actions of growth hormone at the level of the growth plate, occurs early in CKD. It causes severe short stature in most untreated children before the initiation of dialysis (1) and results in major consequences for both physical and psychosocial well-being (2,3). In pediatric patients with CKD stages 2–4, supraphysiologic doses of recombinant growth hormone overcome growth plate resistance to growth hormone, thus increasing growth and improving final adult height (2–4); however, the response to growth hormone in patients with ESRD (CKD stage 5) varies (5–9). The precise mechanism behind decreased responsiveness to growth in dialysis patients is incompletely understood and is probably multifactorial, but because both high and low bone turnover increase the severity of growth retardation (10,11), alterations in trabecular bone turnover may contribute.
Although bone formation rate affects growth velocity, growth hormone therapy may in turn alter trabecular bone histologic features. Indeed, independent of its action on the growth plate, growth hormone worsens the biochemical lesions of secondary hyperparathyroidism and also directly increases osteoblast proliferation and activity, thereby promoting new bone formation and increasing bone turnover (12–16). By contrast, calcitriol, the primary therapeutic agent used to control secondary hyperparathyroidism, blocks the proliferative effects of growth hormone on chondrocytes in young rats with CKD (16). To our knowledge, however, the effects of growth hormone therapy on bone turnover and its interaction with calcitriol in pediatric dialysis patients have not been directly evaluated. Thus, this study was performed to test the hypothesis that growth hormone therapy has a direct effect on bone turnover that is altered by concomitant calcitriol therapy in children treated with continuous cycling peritoneal dialysis.
Materials and Methods
Potential participants were patients with ESRD undergoing continuous cycling peritoneal dialysis between July 1994 and May 1999 at the University of California, Los Angeles (UCLA), who were age 2–21 years. The trial was not registered in the Clinical Trials Registry (http://www.ClinicalTrials.gov) because it was conducted before registration requirements. Exclusion criteria were a history of poor medication adherence, parathyroidectomy within the past 12 months, epiphyseal growth plate closure, or treatment with prednisone or any other immunosuppressive agent. After a 4-week period of withdrawal from vitamin D therapy, patients were admitted to the UCLA General Clinical Research Center; bone biopsy specimens were obtained from the anterior iliac crest using a modified Bordier trephine after double tetracycline labeling (10–15 mg/kg per day, taken orally thrice daily during two 2-day periods separated by a 12-day tetracycline-free interval) (17). Bone quantitative histomorphometry was performed and variables compared with reference values established in children with normal renal function, as described previously (18). The terminology established by the Nomenclature Committee of the American Society for Bone and Mineral Research was used to report all histomorphometric variables (19), and lesions of renal osteodystrophy were categorized according to the recommended TMV (turnover, mineralization, volume) classification system (20).
After bone biopsy, patients were divided into two groups according to bone formation rate per bone surface (BFR/BS): Group 1 (high-turnover renal osteodystrophy) was defined by increased BFR/BS or by the presence of bone marrow fibrosis, and group 2 (low-turnover osteodystrophy) was defined by normal or decreased rates of bone formation without evidence of fibrosis. Patients in each group were then randomly assigned in groups of eight with a 1:1 allocation ratio to treatment with subcutaneous growth hormone therapy or to no growth hormone therapy. All patients in group 1, regardless of randomization, also received intermittent calcitriol therapy alone, whereas all patients in group 2 did not. The study was nonblinded.
For patients with high bone turnover, the initial dose of intraperitoneal calcitriol was 1 μg administered thrice weekly (Monday, Wednesday, and Friday); the dose was then increased in 0.5-μg increments to achieve serum calcium levels of 10–10.5 mg/dl and serum phosphorus levels <6.0 mg/dl. Calcitriol therapy was adjusted according to serum calcium and phosphorus levels; therapy was temporarily withheld if severe hypercalcemia (defined by a serum calcium level >11.0 mg/dl) or hyperphosphatemia (serum phosphorus >7 mg/dl) developed. Calcitriol therapy was resumed after the dose was reduced by 50% when serum calcium and phosphorus levels returned to target values. For all patients with low or high bone turnover randomly assigned to receive growth hormone, the dose was 0.05 μg/kg per day subcutaneously; doses were modified at each visit depending on body weight. All patients were treated with calcium-based binders, and the dialysate calcium concentration was 2.5 mEq/L. Patients underwent therapy for 8 months, after which time bone biopsy was repeated. In the event of kidney transplantation, treatment was terminated early and bone biopsy was performed at the time of transplantation.
Biochemical determinations of serum calcium, phosphorus, alkaline phosphatase, IGF-1, and parathyroid hormone (PTH) levels were obtained at the time of both bone biopsies and at monthly intervals during the study. Serum samples for PTH and IGF-1 were stored at −70°C and run in batches at the conclusion of the study (IGF-1 ELISA kit, Diagnostic Systems Laboratories, Webster, TX, and first-generation PTH Nichols assay, Nichols Institute Diagnostics, San Juan Capistrano, CA, respectively). Serum calcium, phosphorus, and alkaline phosphatase levels were measured using standard laboratory methods. Growth rates were obtained monthly. Standing heights were measured monthly using a fixed, wall-mounted stadiometer. All height measurements were performed by the same person and were repeated until three consecutive values agreed within 0.2 cm, as previously described (21). For statistical evaluation, height measurements were expressed as standard deviation scores (SDSs), or Z-scores, relative to values corresponding to the 50th percentile of the healthy population for children of the same age and sex according to tables provided by the National Center for Health Statistics (Hyattsville, Maryland). Delta Z-scores for height were calculated from values obtained before and after treatment. The study was approved by the UCLA Human Subject Protection Committee. Informed consent was obtained from all parents, and assent forms were obtained from all patients older than age 8 years.
A recruitment goal of 10 patients per treatment group was based on achieving 87% power to detect an anticipated observed effect size of 1.0 SD unit difference in means between treatment groups with an alpha of 0.05. Prevalence was reported as percentage (95% confidence intervals [CIs]). For biochemical and bone histomorphometric variables, means and SDs were reported for normally distributed variables; medians and interquartile ranges (IQRs) were used for variables with non-normal distributions. Differences between baseline and final bone histomorphometric variables were compared using paired t tests, ANOVA with contrasts, and Wilcoxon signed-rank sums. A mixed model was used to evaluate the effects of underlying bone histologic features, treatment group, time on therapy, and biochemical measures. Statistical analysis of the data were performed using SAS software (SAS Institute, Inc., Cary, NC).
Results
Patient Characteristics
A total of 36 patients treated with peritoneal dialysis underwent initial bone biopsy (20 with high turnover, 16 with low turnover). Three were lost to follow-up (one with high turnover, two with low turnover) shortly after randomization; thus, 33 patients with a median age of 11.7 (IQR, 7.6, 14.1) years completed the study. Table 1 summarizes the clinical characteristics of the study population at baseline. Overall, 45% of the patients were male, and 52% were prepubertal; they had been undergoing dialysis for a median of 0.4 (IQR, 0.1, 4.3) year. Nineteen patients had high-turnover osteodystrophy (group 1) and were therefore randomly assigned to growth hormone plus calcitriol (n=8) or to calcitriol alone (n=11). Fourteen patients with low-turnover osteodystrophy (group 2) were randomly assigned to growth hormone treatment (n=7) or no growth hormone treatment (n=7). During the trial, six patients (four in group 1 and two in group 2) withdrew early because of renal transplantation. This occurred within the first 4 months of the trial for two individuals (one in group 1 and one in group 2) and within the last 4 months of the trial for the remaining four.
Table 1.
Baseline clinical characteristics
| Variable | Low-Normal Bone Turnover, No GH | Low-Normal Bone Turnover, GH | High Bone Turnover, Calcitriol, No GH | High Bone Turnover, Calcitriol, GH |
|---|---|---|---|---|
| Patients (n) | 7 | 7 | 11 | 8 |
| Age (yr) | 9.5 (2.8, 14.3) | 8.3 (7.8, 1) | 12.3 (11.7, 14.5) | 13.9 (11.4, 15.5) |
| Male/female (n/n) | 2/5 | 2/5 | 7/4 | 4/4 |
| Ethnicity | 1 | |||
| White | 1 | 3 | 1 | 2 |
| Black | 6 | 3 | 9 | 1 |
| Hispanic | 1 | 4 | ||
| Asian | 1 | |||
| Time on dialysis (yr) | 0.6 (0.4, 1.2) | 0.3 (0.2, 0.8) | 0.4 (0.3, 0.6) | 0.4 (0.3, 0.8) |
| Patients receiving calcitriol before the 4-week washout, n (%) | 3 (43) | 2 (29) | 3 (43) | 2 (25) |
| Prepubertal patients, n (%)a | 4 (57) | 4 (57) | 5 (45) | 4 (50) |
| Z-score body weight | −1.8 (−2.8, −1.6) | −1.1 (−1.9, −0.7) | −1.2 (−1.9, −0.6) | −1.5 (−2.3, −0.7) |
| Z-score height | −2.6 (−3.9, −1.0) | −2.3 (−2.7, −1.7) | −2.1 (−2.2, −1.3) | −2.3 (−3.3, −1.3) |
Prepubertal patients: Tanner stage 1; pubertal patients: Tanner stage 2–5.
Average calcitriol doses in patients with secondary hyperparathyroidism treated with growth hormone in addition to calcitriol were 1.0±0.7 μg per dose given thrice weekly (average weekly dosing, 3.0±2.1 μg per week) and did not differ from those receiving calcitriol alone (1.2±0.7 μg per dose or 3.6±2.1 μg per week; P=0.32 between groups). During the last half of the trial, when doses were stable, average doses were 1.2±0.9 versus 1.3±0.9 μg per dose (3.6±2.7 versus 3.9±2.7 μg per week), respectively (P=0.63). Adverse events consisted solely of hypercalcemic and hyperphosphatemic episodes. Eight episodes of severe hypercalcemia, defined as a calcium value >11 mg/dl, occurred in two patients, both of whom had low bone turnover at baseline; thus, therapy was not modified in these individuals. Eight episodes of hyperphosphatemia (phosphate >7 mg/dl; four randomly assigned to growth hormone plus calcitriol and four assigned to calcitriol alone) necessitating the temporary cessation of calcitriol therapy occurred in patients with high bone turnover.
Changes in Bone Histomorphometry throughout the Study
Table 2 summarizes the bone histomorphometric variables during the study. Bone turnover decreased from 103.3 (57.0, 173.4) to 60.3 (20.3, 103.7) μm2/μm3 per year in children with high bone turnover who were treated with calcitriol alone (P=0.05) but remained unchanged in those receiving growth hormone combined with calcitriol. In patients with low baseline bone turnover, BFR/BS increased from 15.0 (9.6, 21.8) to 154.6 (23.7, 174.3) μm2/μm3 per year (P=0.03 from baseline) in those receiving growth hormone therapy but remained unchanged in those receiving standard therapy. Overall, BFR/BS was higher in patients receiving growth hormone therapy than in those receiving standard therapy (P=0.05).
Table 2.
Baseline and final bone histomorphometric features
| Bone Measures | Low Bone Turnover | High Bone Turnover | Normal Range | |||||||
|---|---|---|---|---|---|---|---|---|---|---|
| No GH | GH | Calcitriol | Calcitriol + GH | |||||||
| Baseline (n=7) | Final (n=5) | Baseline (n=7) | Final (n=7) | Baseline (n=11) | Final (n=9) | Baseline (n=8) | Final (n=6) | |||
| Turnover | ||||||||||
| Bone formation rate (μm2/μm3 per year)ab | 7.1 (0.0–13.2) | 40.8 (40.1, 74.9) | 15.0 (9.6, 21.8) | 154.6 (23.7, 174.3c) | 103.3 (57.0, 173.4) | 60.3 (20.3, 103.7)c | 84.9 (67.1, 137.3) | 85.9 (75.1, 130.5) | 10–73.4 | |
| Mineralization | ||||||||||
| Osteoid volume (%)a | 4.6 (3.7, 7.0) | 9.5 (9.4, 15.1) | 5.2 (4.0, 8.1) | 10.9 (7.4, 14.9) | 16.3 (13.0, 18.7) | 13.2 (8.3, 18.3) | 12.3 (8.9, 14.1) | 10.6 (9.9, 13.0) | 0.2–5.86 | |
| Osteoid thickness (µm)a | 12.2±3.6 | 13.4±3.1 | 11.1±2.7 | 14.5±4.5 | 18.0±3.6 | 18.2±5.2 | 15.4±4.3 | 17.8±3.9 | 3–13.9 | |
| Osteoid maturation time (d) | 13 (10, >5000) | 12 (11, 13) | 10 (9, 12) | 13 (11, 14) | 12 (7, 16) | 18 (14, 20) | 11 (10, 13) | 16 (14, 19) | 1.9–10.4 | |
| Mineralization lag time (d)ab | 214 (104, >5000) | 44 (31, 95) | 99 (62, 209) | 24 (22, 42)c | 33 (23, 44) | 80 (39, 95)c | 36 (24, 50) | 35 (30, 37) | 4.1–71.7 | |
| Volume | ||||||||||
| Bone volume (%) | 23.9±4.8 | 21.6±6.4 | 24.9±6.5 | 27.3±11.8 | 28.2±6.2 | 30.3±8.3 | 24.1±5.7 | 32.9±9.6 | 9.3–22.7 | |
| Trabecular thickness (µm)a | 135±17 | 125±31 | 134±29 | 152±46 | 149±29 | 161±29 | 142±22 | 175±26 | 72–177 | |
| Trabecular separation (µm) | 457±82 | 483±154 | 420±114 | 459±144 | 405±107 | 386±102 | 491±243 | 385±168 | 299–587 | |
| Trabecular number per mm2 | 1.72±0.28 | 1.73±0.48 | 1.90±0.51 | 1.69±0.35 | 1.87±0.38 | 1.88±0.33 | 1.75±0.50 | 1.90±0.50 | 1.3–2.7 | |
Normally distributed values are displayed as means ± SD; skewed data are displayed as median (interquartile range). GH, growth hormone.
Significant (P<0.05) baseline difference between patients with high and low bone turnover.
Significant (P≤0.05) difference, adjusting for baseline values, between GH treatment and no GH treatment.
Significant (P≤0.05) difference between baseline and 8 months.
Static measures of mineralization, including osteoid volume/bone volume, osteoid surface/bone surface, and osteoid thickness were lower in patients with low bone turnover than in those with high bone turnover (P<0.001 for all three measures) but did not change during therapy, irrespective of baseline bone histologic features and treatment group. Mineralization lag time increased from 33 (23, 44) to 80 (39, 95) days in patients with high bone turnover receiving calcitriol alone (P=0.03), but not in those treated with growth hormone; similarly, a decrease in mineralization lag time was observed in children with low baseline bone turnover who received growth hormone from 99 (62, 209) to 24 (22, 42) days (P=0.03), whereas no change was observed in those receiving standard therapy. Overall, final mineralization lag time was lower in patients treated with growth hormone than in those receiving standard therapy, irrespective of baseline bone histologic features (P=0.03). Osteoid maturation time did not change during therapy.
At baseline, measures of bone volume, including bone volume/tissue volume and trabecular thickness, were normal or increased in all patients. These measures remained stable throughout therapy, and no differences were observed between treatment groups.
Biochemical Determinations
Baseline and final biochemical values for the four treatment groups are displayed in Table 3. Baseline serum calcium levels were higher in patients with low bone turnover than in those with high bone turnover (9.8±0.7 mg/dl versus 9.0±1.0 mg/dl in patients with low versus high turnover, respectively; P=0.04) and remained stable throughout the study in all patients; no patients experienced severe hypercalcemia. Any degree of hypercalcemia, as defined by a serum calcium level >10.4 mg/dl, was present in five individuals at baseline: three with secondary hyperparathyroidism and two with adynamic/normal bone. Throughout the study, four episodes of hypercalcemia (4% [95% CI, 1%–10%] of values) were observed in patients with secondary hyperparathyroidism treated with growth hormone and calcitriol and six episodes (9% [95% CI, 3%–18%] of values) were observed in those treated with growth hormone alone. By contrast, hypercalcemia was more common in patients with adynamic bone. Thirteen episodes (20% [95% CI, 11%–31%] of values) of hypercalcemia were observed in patients with adynamic bone treated with growth hormone compared with only four episodes (5% [95% CI, 1%–12%] of values) in patients with adynamic bone not receiving growth hormone therapy. Eight episodes of severe hypercalcemia, defined as a value >11 mg/dl, were observed during the study; there was no difference between groups in number of episodes of severe hypercalcemia.
Table 3.
Baseline and final biomarkers
| Serum Biochemical Measures | No GH Therapy | GH Therapy | ||
|---|---|---|---|---|
| Initial | Final | Initial | Final | |
| High bone turnover on initial bone biopsy | ||||
| Patients (n) | 11 | 9 | 8 | 6 |
| Corrected calcium (mg/dl)a | 9.4±0.9 | 9.6±0.5 | 9.9±1.1 | 9.3±0.8 |
| Phosphorus (mg/dl) | 5.8±0.9 | 5.7±0.8 | 5.2±0.9 | 7.0±2.5 |
| Alkaline phosphatase (IU/L)a | 436 (374, 703) | 371 (198, 508)b | 278 (253, 328) | 370 (250, 580)b |
| PTH (pg/ml)a | 728 (411, 1078) | 335 (152, 867) | 533 (491, 669) | 579 (578, 767) |
| IGF-1 (ng/ml) | 356 (133, 435) | 235 (182, 405) | 254 (235, 380) | 319 (182, 329) |
| Low/normal bone turnover on initial bone biopsy | ||||
| Patients (n) | 7 | 5 | 7 | 7 |
| Corrected calcium (mg/dl)a | 9.6±0.7 | 9.4±0.5 | 10.0±0.7 | 9.1±1.1 |
| Phosphorus (mg/dl) | 6.5±1.8 | 7.4±2.2 | 5.1±1.8 | 6.5±1.2b |
| Alkaline phosphatase (IU/L)a | 306 (185, 434) | 332 (170, 465)b | 185 (139, 303) | 285 (240, 392)b |
| PTH (pg/ml)a | 136 (62, 332) | 755 (110, 803) | 119 (89, 125) | 775 (310, 1361)b |
| IGF-1 (ng/ml) | 229 (100, 462) | 313 (104, 522) | 191 (159, 320) | 490 (292, 554)b |
Normally distributed values are displayed as mean ± SD; skewed data are displayed as median (interquartile range).
P<0.05 between patients with high turnover and patients with low-normal turnover at baseline.
P<0.05 from baseline.
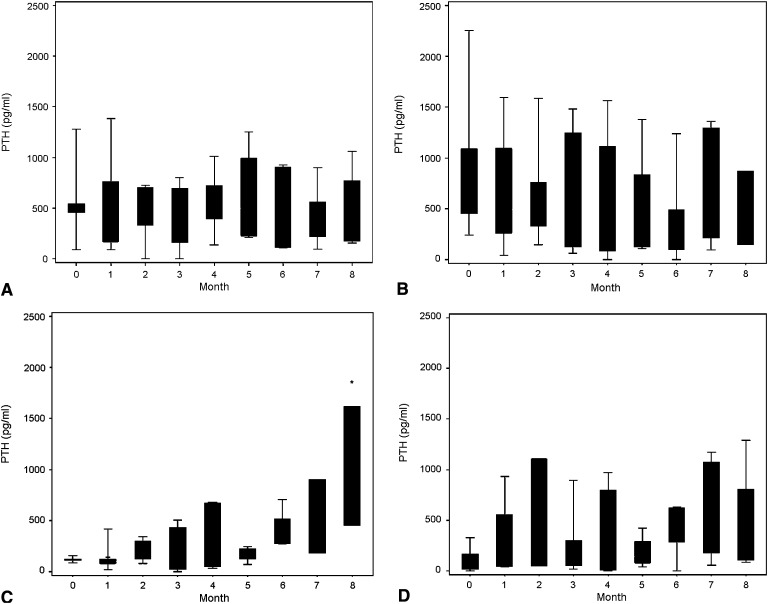
Serum phosphorus levels did not differ based on bone histologic features at baseline (5.8±1.9 mg/dl for those with low bone turnover versus 5.6±0.9 mg/dl for those with high bone turnover group; P=0.79), whereas IGF-1 values were lower in patients with low bone turnover (P=0.04 in low- versus high-turnover groups, respectively). Serum phosphate concentrations did not differ between groups throughout the study (P=0.14 between groups); however, IGF-1 increased in patients with low bone turnover who received growth hormone (P=0.01) but did not change in the other groups. Alkaline phosphatase and PTH values were lower at baseline in patients with low bone turnover than in those with high bone turnover (P=0.03 and P<0.001 between histologic classifications for alkaline phosphatase and PTH, respectively). Of note, baseline PTH values in patients with high bone turnover did not differ by treatment group (P=0.77), and no significant differences were observed during therapy, regardless of whether those patients received calcitriol therapy alone or were treated with both calcitriol and growth hormone. Similarly, baseline PTH levels were similar in the two groups of patients with low bone turnover (P=0.75), and, although PTH values significantly increased only in patients with low bone turnover receiving growth hormone therapy (P=0.03), values did not differ between the two treatment groups during therapy (Figure 1).
Figure 1.
Change in serum parathyroid hormone (PTH) levels throughout the study. No significant changes from baseline or difference between groups were observed in patients with high bone turnover receiving growth hormone plus calcitriol (A) versus those treated with calcitriol alone (B). Serum PTH levels increased from baseline in patients with low bone turnover receiving growth hormone therapy (C) but not in those receiving standard treatment alone (D). PTH values did not differ between the two groups of patients with low bone turnover. *P<0.05 for change from baseline. Boxes represent the interquartile range; whiskers represent the minimum and maximum.
Growth
At baseline, Z-scores for statural height and body weight based on bone histologic features did not differ. Overall, height SDS increased in patients who were treated with growth hormone therapy while remaining unchanged in patients not receiving growth hormone, with an estimated difference in change in height SDS between groups of 0.324±0.076 SDs (P<0.001 between groups). Underlying bone histologic features did not affect changes in height Z score.
Discussion
This study demonstrated that growth hormone therapy resulted in an increase in bone turnover in patients with low bone turnover—patients who were not receiving concomitant calcitriol therapy—while not altering bone formation in patients with high bone turnover who received growth hormone in conjunction with calcitriol. Although baseline serum calcium levels were higher in patients with low bone turnover than in those with high bone turnover, treatment with growth hormone, with or without calcitriol, did not alter serum calcium levels. By contrast, serum phosphorus, PTH, alkaline phosphatase, and IGF-1 levels increased in patients with low bone turnover who received growth hormone therapy. Linear growth increased in patients treated with growth hormone, irrespective of underlying bone histologic features.
Calcitriol is effective at controlling the skeletal lesions of secondary hyperparathyroidism in patients treated with maintenance dialysis, yet the effect of calcitriol in combination with growth hormone on bone turnover in dialysis patients and the role that preexisting bone disease plays in mediating response to growth hormone remain unknown. Moreover, previous data suggested that growth hormone therapy may exacerbate secondary hyperparathyroidism, although the consequences for these increased values on the skeleton were previously unknown. This lack of data are described in the Kidney Disease Outcomes Quality Initiative (KDOQI) guidelines (22), which state that “there is insufficient clinical research on rhGH [recombinant human growth hormone] therapy and bone disease. Guidelines for monitoring PTH, calcium, phosphorus, alkaline phosphatase, and X-rays are based on expert opinion, not firm clinical evidence.” These guidelines also highlight the “absence of firm clinical data supporting the levels of PTH upon which cessation and reinitiation of GH are based.”
In this study, bone turnover increased in all patients with low baseline bone turnover, although a statistically significant increase was observed only in those receiving growth hormone therapy, despite similar PTH levels between the two groups of patients with low bone turnover. Consistent with both the 2005 KDOQI and the 2006 European guidelines, which highlight the potential for growth hormone therapy to increase circulating PTH values (22,23), PTH increased in patients with low bone turnover who were treated with growth hormone; this subset of patients did not receive concomitant calcitriol therapy. Of note, however, final PTH values in this subset of patients were similar to those of patients with low bone turnover who did not receive growth hormone therapy, despite marked differences in final rates of bone formation. This finding suggests that the direct effects of growth hormone on bone turnover distort the relationship between bone formation rate and PTH in patients treated with maintenance dialysis. In patients with high baseline bone turnover, calcitriol doses and PTH levels were similar between patients receiving a combination of growth hormone and calcitriol and those receiving calcitriol alone, suggesting that the biochemical lesions of secondary hyperparathyroidism can be controlled during growth hormone therapy with the use of active vitamin D sterols. However, bone formation rates decreased only in patients who were treated with calcitriol alone while remaining elevated in those receiving combined therapy with growth hormone and calcitriol. This finding further confirms the disrupted relationship between PTH values and bone turnover during growth hormone therapy.
Although target serum PTH levels are usually recommended to guide therapeutic decisions in CKD/mineral and bone disorder, PTH varied widely both in patients with low bone turnover and those with high bone turnover, with ranges of PTH overlapping significantly both at baseline and throughout therapy. This overlap may have been due to various causes; indeed, values of bioactive PTH in dialysis patients are overestimated by current assays because of the cross-reactivity between the full-length PTH molecule and its different fragments (24), and treatments for secondary hyperparathyroidism disrupt the relationship between PTH and bone histologic features (25,26). Regardless of their cause, together these findings suggest that (1) skeletal lesions of secondary hyperparathyroidism persist in patients treated with a combination of growth hormone and calcitriol while growth hormone increases bone turnover in patients with low bone formation rates, (2) growth hormone disrupts the relationship between circulating PTH levels and bone turnover, and (3) PTH alone cannot correctly predict the underlying bone disease in children treated with maintenance dialysis and should be used cautiously for therapeutic decisions.
The increase in bone formation rate from 7 to 41 μm2/mm3 per year in patients with low turnover not receiving growth hormone therapy may represent a biologically important increase in bone turnover with cessation of calcitriol therapy; however, this final value is within the range of normal for bone formation rate, and the small number of patients in the study probably precluded a statistically significant result. Whether patients with normal bone turnover should receive calcitriol therapy is beyond the scope of this study, but the overtly elevated rates of bone formation observed in patients with adynamic bone disease receiving growth hormone therapy (an effect so marked it was seen even with the small number of patients in this study) suggests that calcitriol therapy may be warranted in these patients. However, it is not clear from the current data whether the prevention and treatment of adynamic bone disease should be considered the same issue as treatment of high-turnover renal osteodystrophy. Indeed, it is likely that detection, prevention, and treatment of adynamic bone disease must all be evaluated in light of many clinical factors, including not only PTH levels but also underlying disease, concurrent therapies for underlying disease (such as steroids), and concurrent therapies for bone disease (including calcium-containing or calcium-free phosphate binders, calcitriol and vitamin D sterols, and calcimimetic agents), as well as the presence or absence of vascular calcifications.
In this study, although overall growth velocity improved in all patients treated with growth hormone, circulating IGF1 levels significantly increased only in patients with low bone turnover treated with growth hormone and not in those with high bone turnover. This discrepancy may be in part explained by two different factors. Overall, patients with low bone turnover tended to be younger than those with high bone turnover, although the proportion of prepubertal patients did not differ between patients with high and low bone turnover. Because IGF-1 levels vary more with pubertal development than with age, assessing the effect of age per se on such levels in dialysis patients is fraught with difficulty. Moreover, growth hormone itself has been shown to directly affect osteoblastic activity—potentially through local skeletal IGF-1 production and not exclusively through hepatically produced IGF-1 (27). As a result, circulating IGF-1 levels are an unreliable marker for monitoring growth hormone efficacy in this patient population. Finally, because this study was designed to assess the effect of growth hormone therapy on bone histologic features, with growth as a secondary endpoint, and because many pediatric patients with ESRD undergo renal transplantation within a year of initiating dialysis, we chose an 8-month therapeutic time frame to avoid excessive dropouts from transplantation while still achieving meaningful data on bone histologic features. More robust changes in the secondary endpoints of growth and in IGF-1 levels may have been observed in a study of longer duration, and these issues warrant further evaluation.
The primary limitation of this study is its small sample size, and, indeed, the number of patients with low bone turnover in the study was smaller than anticipated; however, the effect of growth hormone in this subset of patients was so marked as to yield a significant increase in bone turnover and growth velocity despite the small numbers. This study also presents biochemical data and bone histologic features exclusively from patients receiving calcitriol as an active vitamin D metabolite and calcium carbonate as a phosphate binder. Current data suggest that hyperphosphatemia can be effectively treated and hypercalcemia avoided in patients while bone formation rates are controlled when pediatric patients are treated with non–calcium-containing binders, such as sevelamer, in combination with active vitamin D sterols. However, this form of therapy necessitates increased doses of vitamin D to achieve a similar suppression of bone formation rates (26), and the potential interaction between these increased doses and growth hormone therapy on the skeleton warrants further investigation.
The intraperitoneal method of calcitriol administration used in the current study may also limit the data interpretation. Although the bioavailability of intraperitoneal calcitriol, as determined from the 24-hour area-under-the-curve measurements, is similar to the bioavailability of orally administered calcitriol, intravenous administration of calcitriol results in a 50%–60% greater bioavailability, due primarily to higher calcitriol concentrations in the first 6 hours after intravenous administration (28). Thus, similar doses given by the intravenous route may result in greater suppression of both PTH values and bone turnover, and further studies are warranted to assess whether these differences alter the interaction with growth hormone therapy, both at the level of the parathyroid gland and at the level of bone. Finally, PTH in this study was measured with the Nichols assay, which was historically the standard assay used in clinical practice. Although this assay is no longer available, values obtained by the current Immutopics first-generation assay are highly correlated with values obtained by the old Nichols assay (29). Myriad other PTH assays are now routinely used in clinical practice, and PTH determinations by these different assays vary widely, even when tested on the same samples (30). This issue further strengthens the conclusion that PTH alone cannot correctly predict the underlying bone disease in children treated with maintenance dialysis and should be used cautiously for therapeutic decision-making.
In conclusion, to our knowledge this is the first study evaluating the direct effect of recombinant human growth hormone therapy on the skeleton across the spectrum of renal osteodystrophy in pediatric patients treated with maintenance peritoneal dialysis. Therapy with growth hormone enhanced bone turnover in patients with baseline low bone turnover, while skeletal lesions of secondary hyperparathyroidism persisted in patients with high bone turnover at baseline, despite the concomitant use of calcitriol. Unfortunately, biochemical measures were poor predictors of skeletal response. Thus, the addition of growth hormone to therapy for pediatric renal osteodystrophy may help to prevent the development of adynamic bone but may also potentiate the lesions of secondary hyperparathyroidism. Future longitudinal studies are needed to evaluate the effect of growth hormone therapy on final height, fracture risk, bone deformities, and puberty in children with ESRD.
Disclosures
I.B.S. has received honoraria from Genzyme, Abbott, Johnson & Johnson, and Cytochroma.
Acknowledgments
This work was supported in part by U.S. Public Health Service grants DK-67563, DK-35423, DK-51081, DK-073039, UL1 RR-033176, and UL1TR000124 and funds from the Casey Lee Ball Foundation.
J.B. received educational grants from l’Académie Française (Jean Walter Zellidja), la Réunion Pédiatrique de la Région Rhône Alpes (RPRRA), la Société Française de Pédiatrie (Evian), la Fondation pour la Recherche Médicale, and the Philippe Foundation.
Footnotes
Published online ahead of print. Publication date available at www.cjasn.org.
References
- 1.North American Pediatric Renal Transplant Cooperative Study (NAPRTCS): 2005 Annual Report. 2005. Available at: https://web.emmes.com/study/ped/annlrept/annlrept2005.pdf Accessed January 2012
- 2.Haffner D, Schaefer F, Nissel R, Wühl E, Tönshoff B, Mehls O, German Study Group for Growth Hormone Treatment in Chronic Renal Failure : Effect of growth hormone treatment on the adult height of children with chronic renal failure. N Engl J Med 343: 923–930, 2000 [DOI] [PubMed] [Google Scholar]
- 3.Vimalachandra D, Hodson EM, Willis NS, Craig JC, Cowell C, Knight JF: Growth hormone for children with chronic kidney disease. Cochrane Database Syst Rev (3): CD003264, 2006 [DOI] [PubMed] [Google Scholar]
- 4.Hokken-Koelega AC, Stijnen T, de Muinck Keizer-Schrama SM, Wit JM, Wolff ED, de Jong MC, Donckerwolcke RA, Abbad NC, Bot A, Blum WF, et al. : Placebo-controlled, double-blind, cross-over trial of growth hormone treatment in prepubertal children with chronic renal failure. Lancet 338: 585–590, 1991 [DOI] [PubMed] [Google Scholar]
- 5.Nissel R, Lindberg A, Mehls O, Haffner D, Pfizer International Growth Database (KIGS) International Board : Factors predicting the near-final height in growth hormone-treated children and adolescents with chronic kidney disease. J Clin Endocrinol Metab 93: 1359–1365, 2008 [DOI] [PubMed] [Google Scholar]
- 6.Lanes R, Gunczler P, Orta N, Bosquez M, Scovino R, Dominguez L, Esaa S, Weisinger JR: Changes in bone mineral density, growth velocity and renal function of prepubertal uremic children during growth hormone treatment. Horm Res 46: 263–268, 1996 [DOI] [PubMed] [Google Scholar]
- 7.Boot AM, Nauta J, de Jong MC, Groothoff JW, Lilien MR, van Wijk JA, Kist-van Holthe JE, Hokken-Koelega AC, Pols HA, de Muinck Keizer-Schrama SM: Bone mineral density, bone metabolism and body composition of children with chronic renal failure, with and without growth hormone treatment. Clin Endocrinol (Oxf) 49: 665–672, 1998 [DOI] [PubMed] [Google Scholar]
- 8.Crompton CH, Australian and New Zealand Paediatric Nephrology Association : Long-term recombinant human growth hormone use in Australian children with renal disease. Nephrology (Carlton) 9: 325–330, 2004 [DOI] [PubMed] [Google Scholar]
- 9.Müller-Wiefel D, Frisch H, Tulassay T, Bell L, Zadik Z: Treatment of growth failure with growth hormone in children with chronic kidney disease: An open-label long-term study. Clin Nephrol 74: 97–105, 2010 [DOI] [PubMed] [Google Scholar]
- 10.Kuizon BD, Goodman WG, Jüppner H, Boechat I, Nelson P, Gales B, Salusky IB: Diminished linear growth during intermittent calcitriol therapy in children undergoing CCPD. Kidney Int 53: 205–211, 1998 [DOI] [PubMed] [Google Scholar]
- 11.Chesney RW, Moorthy AV, Eisman JA, Jax DK, Mazess RB, DeLuca HF: Increased growth after long-term oral 1alpha,25-vitamin D3 in childhood renal osteodystrophy. N Engl J Med 298: 238–242, 1978 [DOI] [PubMed] [Google Scholar]
- 12.Baroncelli GI, Bertelloni S, Sodini F, Saggese G: Acquisition of bone mass in normal individuals and in patients with growth hormone deficiency. J Pediatr Endocrinol Metab 16[Suppl 2]: 327–335, 2003 [PubMed] [Google Scholar]
- 13.Murray RD, Adams JE, Shalet SM: Adults with partial growth hormone deficiency have an adverse body composition. J Clin Endocrinol Metab 89: 1586–1591, 2004 [DOI] [PubMed] [Google Scholar]
- 14.Colao A, Di Somma C, Pivonello R, Loche S, Aimaretti G, Cerbone G, Faggiano A, Corneli G, Ghigo E, Lombardi G: Bone loss is correlated to the severity of growth hormone deficiency in adult patients with hypopituitarism. J Clin Endocrinol Metab 84: 1919–1924, 1999 [DOI] [PubMed] [Google Scholar]
- 15.Salusky IB, Goodman WG: Growth hormone and calcitriol as modifiers of bone formation in renal osteodystrophy. Kidney Int 48: 657–665, 1995 [DOI] [PubMed] [Google Scholar]
- 16.Sanchez CP, Goodman WG, Brandli D, Goldenhersh M, Murray C, Carlton E, Hahn T, Salusky IB: Skeletal response to recombinant human growth hormone (rhGH) in children treated with long-term corticosteroids. J Bone Miner Res 10: 2–6, 1995 [DOI] [PubMed] [Google Scholar]
- 17.Hernandez JD, Wesseling K, Pereira R, Gales B, Harrison R, Salusky IB: Technical approach to iliac crest biopsy. Clin J Am Soc Nephrol 3[Suppl 3]: S164–S169, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Sanchez CP, Salusky IB, Kuizon BD, Ramirez JA, Gales B, Ettenger RB, Goodman WG: Bone disease in children and adolescents undergoing successful renal transplantation. Kidney Int 53: 1358–1364, 1998 [DOI] [PubMed] [Google Scholar]
- 19.Parfitt AM, Drezner MK, Glorieux FH, Kanis JA, Malluche H, Meunier PJ, Ott SM, Recker RR, Report of the ASBMR Histomorphometry Nomenclature Committee : Bone histomorphometry: Standardization of nomenclature, symbols, and units. J Bone Miner Res 2: 595–610, 1987 [DOI] [PubMed] [Google Scholar]
- 20.Moe S, Drüeke T, Cunningham J, Goodman W, Martin K, Olgaard K, Ott S, Sprague S, Lameire N, Eknoyan G, Kidney Disease: Improving Global Outcomes (KDIGO) : Definition, evaluation, and classification of renal osteodystrophy: A position statement from Kidney Disease: Improving Global Outcomes (KDIGO). Kidney Int 69: 1945–1953, 2006 [DOI] [PubMed] [Google Scholar]
- 21.Salusky IB, Fine RN, Nelson P, Blumenkrantz MJ, Kopple JD: Nutritional status of children undergoing continuous ambulatory peritoneal dialysis. Am J Clin Nutr 38: 599–611, 1983 [DOI] [PubMed] [Google Scholar]
- 22.National Kidney Foundation : K/DOQI clinical practice guidelines for bone metabolism and disease in children with chronic kidney disease. Am J Kidney Dis 46: S1–S121, 2005 [PubMed] [Google Scholar]
- 23.Klaus G, Watson A, Edefonti A, Fischbach M, Rönnholm K, Schaefer F, Simkova E, Stefanidis CJ, Strazdins V, Vande Walle J, Schröder C, Zurowska A, Ekim M, European Pediatric Dialysis Working Group (EPDWG) : Prevention and treatment of renal osteodystrophy in children on chronic renal failure: European guidelines. Pediatr Nephrol 21: 151–159, 2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Lepage R, Roy L, Brossard JH, Rousseau L, Dorais C, Lazure C, D’Amour P: A non-(1-84) circulating parathyroid hormone (PTH) fragment interferes significantly with intact PTH commercial assay measurements in uremic samples. Clin Chem 44: 805–809, 1998 [PubMed] [Google Scholar]
- 25.Goodman WG, Ramirez JA, Belin TR, Chon Y, Gales B, Segre GV, Salusky IB: Development of adynamic bone in patients with secondary hyperparathyroidism after intermittent calcitriol therapy. Kidney Int 46: 1160–1166, 1994 [DOI] [PubMed] [Google Scholar]
- 26.Wesseling-Perry K, Pereira RC, Sahney S, Gales B, Wang HJ, Elashoff R, Jüppner H, Salusky IB: Calcitriol and doxercalciferol are equivalent in controlling bone turnover, suppressing parathyroid hormone, and increasing fibroblast growth factor-23 in secondary hyperparathyroidism. Kidney Int 79: 112–119, 2011 [DOI] [PubMed] [Google Scholar]
- 27.Courtland HW, Sun H, Beth-On M, Wu Y, Elis S, Rosen CJ, Yakar S: Growth hormone mediates pubertal skeletal development independent of hepatic IGF-1 production. J Bone Miner Res 26: 761–768, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Salusky IB, Goodman WG, Horst R, Segre GV, Kim L, Norris KC, Adams JS, Holloway M, Fine RN, Coburn JW: Pharmacokinetics of calcitriol in continuous ambulatory and cycling peritoneal dialysis patients. Am J Kidney Dis 16: 126–132, 1990 [DOI] [PubMed] [Google Scholar]
- 29.Wesseling-Perry K, Harkins GC, Wang HJ, Sahney S, Gales B, Elashoff RM, Jüppner H, Salusky IB: Response of different PTH assays to therapy with sevelamer or CaCO3 and active vitamin D sterols. Pediatr Nephrol 24: 1355–1361, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Joly D, Drueke TB, Alberti C, Houillier P, Lawson-Body E, Martin KJ, Massart C, Moe SM, Monge M, Souberbielle JC: Variation in serum and plasma PTH levels in second-generation assays in hemodialysis patients: a cross-sectional study. Am J Kid Dis. 51: 987—995, 2008 [DOI] [PubMed] [Google Scholar]