Abstract
Several clinically available cardiac biomarkers have established their prognostic value in patients with acute coronary syndromes. However, their relative prognostic significance in stable subjects has not been prospectively validated, either individually or in combination. The aim of this study was to evaluate the extent to which B-type natriuretic peptide, myeloperoxidase, and high-sensitivity C-reactive protein alone or together could be prognostic biomarkers in 3,635 consecutive stable patients without acute coronary syndrome who underwent elective diagnostic coronary angiography. After adjusting for traditional risk factors and renal function, each of the markers monitored was a significant predictor of incident major adverse cardiovascular events (death, nonfatal myocardial infarction, and stroke) over 3 years. A cardiac biomarker score based on the sum total of “positive” biomarkers provided independent prediction of future risk for incident major adverse cardiovascular events at 3 years (hazard ratio [HR] 7.61, 95% confidence interval [CI] 4.98 to 11.65, p <0.001), even after adjusted for traditional risk factors (HR 6.11, 95% CI 3.98 to 9.38, p <0.001). A positive cardiac biomarker score remained a strong and independent predictor of 3-year risk for major adverse cardiovascular events among those with normal glycemic control (HR 4.24, 95% CI 1.96 to 9.18, p <0.001), those with prediabetes (HR 7.62, 95% CI 3.87 to 15.01, p <0.001), and those with diabetes (HR 5.61, 95% CI 2.55 to 12.33, p <0.001), as well as within subjects without significant angiographic evidence of coronary artery disease (HR 10.82, 95% CI 3.82 to 30.6, p <0.001). In conclusion, an integrated assessment of cardiac biomarkers may provide independent prognostic value for long-term adverse clinical events in stable cardiac patients.
Increasingly, cardiac biomarkers have provided important information in predicting short-term and long-term risk profiles in patients with acute coronary syndromes, particularly when used in combination.1 Several clinically available cardiac biomarkers, including B-type natriuretic peptide (BNP),2 myeloperoxidase (MPO),3 and high-sensitivity C-reactive protein (hsCRP),4 provide incremental prognostic value in patients with acute coronary syndromes, alone or in combination. Their ability to predict cardiovascular risk has been postulated, as they reflect underlying biomarkers of myocardial dysfunction, plaque vulnerability, and systemic inflammation, respectively.5 However, the clinical utility of such biomarkers simultaneously in a stable, nonacute patient cohort is less well established. We hypothesized that simultaneous assessment of these clinically available cardiac biomarkers to produce a risk score (composed of the sums of “positive” biomarkers on the basis of established cutoff values) would provide incremental prognostic insight into predicting future adverse cardiovascular outcomes. As there is an evolving understanding of patients with diabetes and prediabetes being at heightened cardiovascular risk, we further analyzed the prognostic utility of these cardiac biomarkers across the spectrum of glycemic control.
Methods
We prospectively evaluated 3,635 consecutively consenting subjects who underwent elective cardiac catheterization recruited from 2001 to 2006 without evidence of myocardial infarction (cardiac troponin I <0.03 ng/ml). All participants gave written informed consent, and the institutional review board of the Cleveland Clinic approved the study protocol. The Framingham risk score was calculated for each subject on the basis of Adult Treatment Panel III guidelines.6 An estimate of creatinine clearance was calculated using the Cockcroft-Gault equation. Coronary artery disease was defined as any clinical history of myocardial infarction, percutaneous coronary intervention, coronary artery bypass surgery, or angiographic evidence of coronary artery disease (≥50% stenosis) in ≥1 major coronary artery. Glycemic status and clinical definition of diabetes mellitus, “prediabetes,” and nondiabetes were defined by the latest practice guidelines on the basis of fasting glucose and glycosylated hemoglobin (HbA1c) levels (fasting glucose <100 mg/dl and HbA1c <5.7% for normal subjects; fasting glucose ≥126 mg/dl or HbA1c ≥6.5% or currently taking glucose-lowering medications for diabetes mellitus; neither normal nor diabetes mellitus for prediabetes).7 Adjudicated outcomes were ascertained over the ensuing 3 years for all subjects after enrollment. The prospective determination of clinical outcomes was made by the research personnel contacting participants independent of study investigators, with prespecified criteria and confirmation by review of documentation independent of the investigators. Major adverse cardiovascular events (MACEs) were defined as death, nonfatal myocardial infarction, or nonfatal cerebrovascular accident after enrollment. Blood samples were collected before the administration of heparin, placed on ice, aliquoted, and frozen at −80°F within 2 hours of collection. All laboratory assays, including hsCRP, BNP, MPO, apolipoprotein A1, apolipoprotein B100, and creatinine, were performed using the Abbott ARCHITECT ci8200 platform (Abbott Diagnostics Inc., Abbott Park, Illinois). The intra- and interassay coefficients were 4% and 2.4% for hsCRP, 2.6% and 3.5% for BNP, and 6.2% and 4.1% for MPO, respectively.
Student’s t tests or Wilcoxon’s rank-sum tests for continuous variables and chi-square tests for categorical variables were used to examine the differences between the groups. A cardiac biomarker score (CBS) was given to each group on the basis of whether it had a positive value for each respective biomarker. We used cutoffs for each of the 3 biomarkers (BNP >100 pg/ml, hsCRP >2.0 ng/L, and MPO >322 pmol/L) on the basis of previous cutoffs used for the respective markers, as reported in previous studies.2,4,8 Each of the groups was categorized as 0, 1, 2, or 3 as a measure of how many biomarkers were deemed positive, which was defined as the CBS. Kaplan-Meier analysis with Cox proportional-hazards regression was used for time-to-event analysis to determine hazard ratios (HRs) and 95% confidence intervals (95% CIs) for MACEs. Unadjusted trends for all-cause mortality rates as well as rates of nonfatal myocardial infarction or stroke with increasing quartiles of MPO, hsCRP, and BNP were evaluated using the Cochran-Armitage test using a time-to-event approach. Adjustments were made for individual traditional cardiac risk factors (including age, gender, low-density and high-density lipoprotein cholesterol, systolic blood pressure, former or current cigarette smoking, diabetes mellitus, apolipoprotein B100/apolipoprotein A1 ratio, history of myocardial infarction, and creatinine clearance) to predict incident 3-year risk for MACEs. Net reclassification analysis was performed with the 2 Cox models adjusted for traditional risk factors. Cutoff values for net reclassification index estimation used a ratio of 6:3:1 for low-, medium-, and high-risk categories. All analyses were performed using R version 8.02 (R Foundation for Statistical Computing, Vienna, Austria), and p values <0.05 were considered statistically significant.
Results
Table 1 describes the baseline characteristics of our study population and is stratified according to glycemic status. The median levels of hsCRP, BNP, and MPO were 2.00 pg/ml (interquartile range 0.91 to 4.47), 83 pg/ml (interquartile range 34 to 200), and 103 pmol/L (interquartile range 70 to 195), respectively. All 3 biomarkers were notably elevated in patients with diabetes compared to those with prediabetes or nondiabetes.
Table 1.
Baseline characteristics
| Variable | Whole Cohort (n = 3,635) |
Diabetes Mellitus (n = 1,014) |
Prediabetes (n = 1,529) |
Nondiabetes (n = 1,092) |
p Value |
|---|---|---|---|---|---|
| Age (yrs) | 63 ± 11 | 64 ± 10 | 63 ± 11 | 61 ± 12 | <0.001 |
| Men | 65% | 61% | 70% | 64% | <0.001 |
| Hypertension | 71% | 78% | 70% | 62% | <0.001 |
| History of myocardial infarction | 33% | 35% | 32% | 32% | <0.150 |
| Systolic blood pressure (mm Hg) | 133 (120–146) | 134 (120–149) | 132 (120–145) | 132 (119–147) | <0.015 |
| Low-density lipoprotein cholesterol (mg/dl) | 95 (78–116) | 95 (77–115) | 97 (80–118) | 94 (76–116) | <0.012 |
| High-density lipoprotein cholesterol (mg/dl) | 34 (28–41) | 32 (27–39) | 34 (28–42) | 34 (29–42) | <0.001 |
| Creatinine clearance (ml/min/1.73 m2) | 100 (76–126) | 99 (74–128) | 100 (77–126) | 100 (79–126) | <0.512 |
| Cigarette smoking | 65% | 64% | 68% | 62% | <0.002 |
| Aspirin | 73% | 73% | 74% | 71% | <0.357 |
| β blockers | 61% | 65% | 62% | 56% | <0.001 |
| Angiotensin-converting enzyme inhibitors or angiotensin receptor blockers | 50% | 60% | 47% | 41% | <0.001 |
| Statins | 59% | 63% | 59% | 54% | <0.001 |
| hsCRP (mg/L) | 2.00 (0.91–4.47) | 2.56 (1.13–5.93) | 1.89 (0.86–3.95) | 1.67 (0.83–4.00) | <0.001 |
| BNP (pg/ml) | 83 (34–200) | 93 (40–240) | 78 (32–177) | 83 (32–198) | <0.001 |
| MPO (pmol/L) | 103 (70–195) | 105 (74–186) | 104 (69–201) | 100 (68–194) | <0.199 |
Data are expressed as mean ± SD, as percentages, or as median (interquartile range).
Table 2 represents the prognostic value of individual cardiac biomarkers in our study cohort. All 3 cardiac biomarkers provided incremental risk prediction in our study cohort. After adjusting for traditional risk factors, including Framingham risk factors, log-transformed BNP, hsCRP, and MPO each remained independent predictors of incident MACEs at 3-year follow-up.
Table 2.
Cox proportional-hazards analyses for individual cardiac biomarker and future major adverse cardiovascular events at 3 years
| Variable | Univariate Model |
Multivariate Model* |
||
|---|---|---|---|---|
| HR (95% CI) | p Value | HR (95% CI) | p Value | |
| BNP >100 pg/ml | 2.76 (2.25–3.39) | <0.001 | 2.10 (1.65–2.68) | <0.001 |
| hsCRP >2 ng/L | 2.10 (1.71–2.58) | <0.001 | 1.82 (1.46–2.28) | <0.001 |
| MPO >322 pmol/L | 1.43 (1.12–1.82) | <0.004 | 1.32 (1.02–1.71) | <0.036 |
Adjusted for age, gender, low-density and high-density lipoprotein cholesterol, systolic blood pressure, former or current cigarette smoking, diabetes mellitus, history of myocardial infarction, and creatinine clearance.
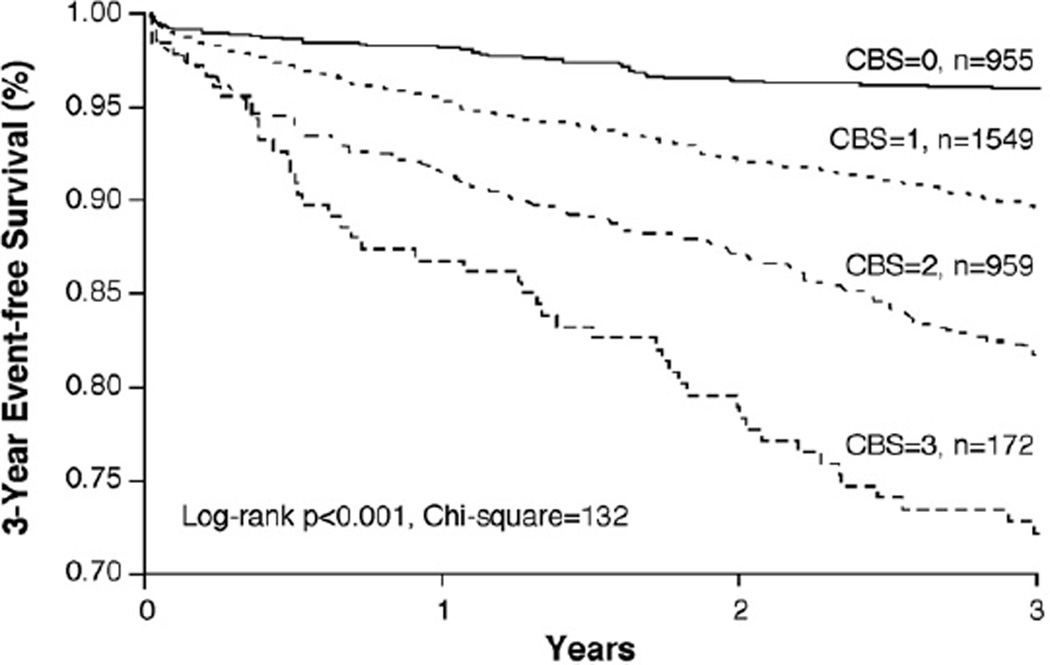
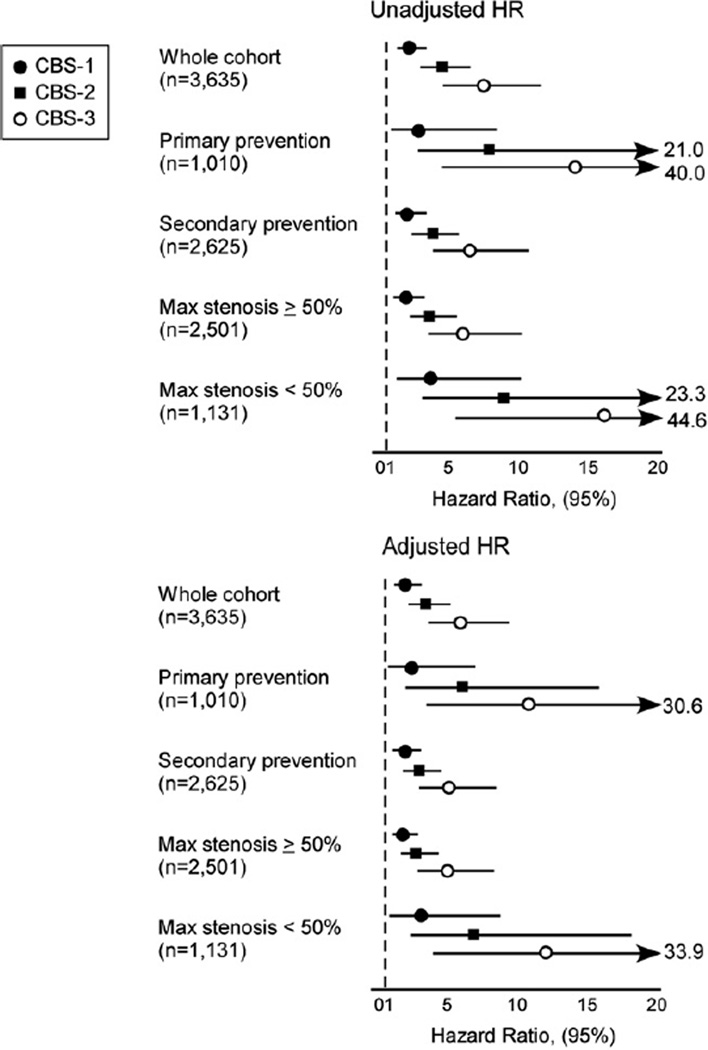
By summing the number of positive cardiac biomarkers, we developed a CBS that integrated the risk profile of our study cohort. As illustrated in Figure 1, the CBS provided incremental prognostic value, as displayed by Kaplan-Meier analysis. As listed in Table 3, the CBS, based on the sum total of positive biomarkers, provided independent prediction of future risk for incident MACEs at 3 years (HR 7.61, 95% CI 4.98 to 11.65, p <0.001), even after adjustment for traditional risk factors (HR 6.11, 95% CI 3.98 to 9.38, p <0.001), in addition to apolipoprotein B100/apolipoprotein A1 ratio (HR 6.11, 95% CI 3.98 to 9.38, p <0.001) (Table 3). The ability of the CBS to provide incremental risk stratification can be seen in subgroups of patients with primary and secondary prevention, as well as those with maximal coronary artery stenoses <50% and ≥50% (Figure 2). A higher CBS predicted future risk for MACEs at 3 years regardless of age, gender, body mass index, diabetes mellitus, hypertension, renal insufficiency, or previous myocardial infarction (p <0.01 for all). Use of the CBS on top of traditional risk factors was also shown to reclassify subjects (net reclassification index 12.86%, p <0.001; integrated discrimination improvement 12.0%, p <0.001; C-statistic 66.9% vs 71.1%, p <0.001).
Figure 1.
Kaplan-Meier analysis of CBS predicting future MACEs at 3-year follow-up.
Table 3.
Cox proportional-hazards analyses of cardiac biomarker score stratified by glycemic status
| Variable | CBS |
|||
|---|---|---|---|---|
| 0 | 1 | 2 | 3 | |
| Whole cohort (n = 3,635) | ||||
| Unadjusted HR | 1 | 2.59 (1.82–3.68)* | 4.72 (3.33–6.69)* | 7.61 (4.98–11.65)* |
| Adjusted HR, model 1 (95% CI) | 1 | 2.27 (1.59–3.23)* | 3.67 (2.58–5.24)* | 6.11 (3.98–9.38)* |
| Adjusted HR, model 2 (95% CI) | 1 | 2.27 (1.59–3.23)* | 3.67 (2.58–5.24)* | 6.11 (3.98–9.38)* |
| MACEs | 39/955 | 159/1,549 | 173/959 | 47/172 |
| Normal (n = 1,014) | ||||
| Unadjusted HR | 1 | 1.78 (0.94–3.37) | 3.23 (1.69–6.16)* | 6.23 (2.9–13.37)* |
| Adjusted HR, model 1 (95% CI) | 1 | 1.43 (0.74–2.75) | 2.37 (1.21–4.64)* | 4.73 (2.18–10.25)* |
| Adjusted HR, model 2 (95% CI) | 1 | 1.36 (0.7–2.61) | 2.23 (1.14–4.39)* | 4.24 (1.96–9.18)* |
| MACEs | 13/290 | 35/445 | 32/230 | 13/49 |
| Prediabetes (n = 1,529) | ||||
| Unadjusted HR | 1 | 2.74 (1.56–4.84)* | 4.75 (2.7–8.38)* | 10.27 (5.24–20.13)* |
| Adjusted HR, model 1 (95% CI) | 1 | 2.4 (1.36–4.23)* | 3.67 (2.06–6.54)* | 7.87 (3.99–15.55)* |
| Adjusted HR, model 2 (95% CI) | 1 | 2.37 (1.35–4.19)* | 3.58 (2–6.41)* | 7.62 (3.87–15.01)* |
| MACEs | 15/427 | 61/654 | 61/379 | 20/69 |
| Diabetes mellitus (n = 1,092) | ||||
| Unadjusted HR | 1 | 3.17 (1.67–6)* | 5.59 (2.98–10.49)* | 6.16 (2.8–13.52)* |
| Adjusted HR, model 1 (95% CI) | 1 | 3.06 (1.61–5.78)* | 4.79 (2.55–9)* | 5.59 (2.54–12.31)* |
| Adjusted HR, model 2 (95% CI) | 1 | 3.05 (1.61–5.77)* | 4.8 (2.55–9.01)* | 5.61 (2.55–12.33)* |
| MACEs | 11/238 | 63/450 | 80/350 | 14/54 |
Model 1 was adjusted for traditional risk factors, including age, gender, systolic blood pressure, low-density lipoprotein cholesterol, high-density lipoprotein cholesterol, and smoking. Model 2 was adjusted for traditional risk factors plus apolipoprotein B100/apolipoprotein A1 ratio.
p <0.001.
Figure 2.
Forest plot of unadjusted and adjusted HRs for predicting future MACEs at 3-year follow-up according to CBS according to subgroups (zero score as reference, adjustments as in Table 3, model 1).
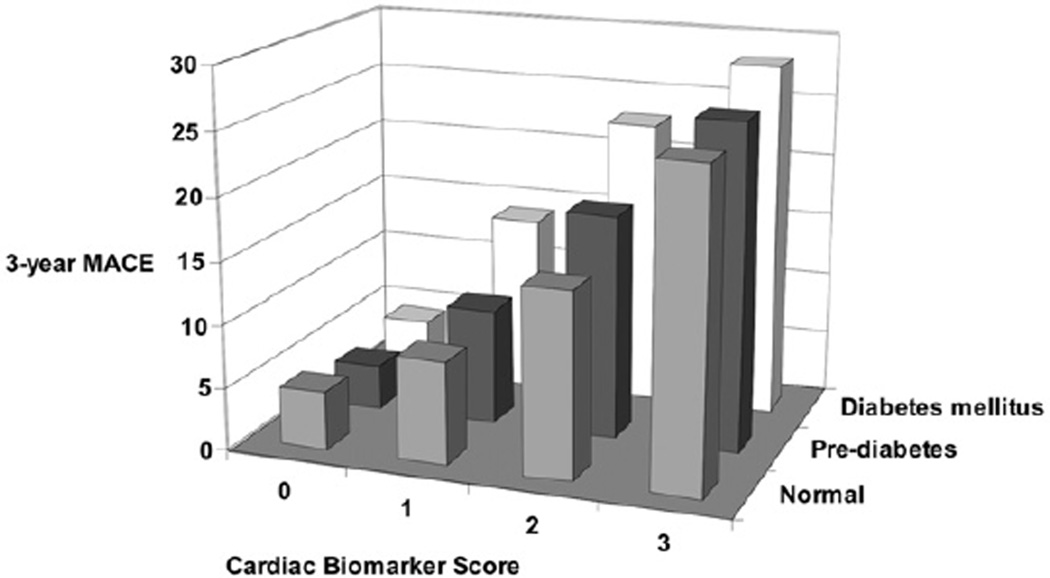
We further analyzed our study cohort according to glycemic status and different subgroups. The capability of the CBS to stratify patients’ risk profiles within subjects with diabetes mellitus, prediabetes, or normal glycemic status (neither prediabetes nor diabetes) on the basis of practice guidelines7 remained robust (Table 3, Figure 3). In a similar manner, after adjustment for HbA1c, the prognostic value of the CBS was preserved. Furthermore, the prognostic value of the CBS was similar regardless of age, gender, body mass index, diabetes mellitus, hypertension, renal insufficiency, or previous myocardial infarction (p <0.01 for all).
Figure 3.
Event rates for future MACEs at 3-year follow-up according to glycemic status.
Discussion
Although previous studies have examined similar multimarker strategies for risk prediction, many of them used biomarkers that are not commonly used or available in the clinical practice settings. The key finding in this study is the incremental prognostic value of all 3 clinically available plasma cardiac biomarkers beyond standard evaluation of classic Framingham risk factors, renal function, and apolipoprotein B100/apolipoprotein A1 ratio in troponin-negative, stable cardiac patients who undergo coronary angiography. We further identified comparable prognostic value within subsets of patients with prediabetes or nondiabetes or those with no significantly obstructive coronary artery disease, further underscoring the potential for a multimarker approach in identifying vulnerable patients within cohorts that may allow targeted risk factor modifications and more aggressive preventive interventions.
HsCRP is the most common systemic inflammatory biomarker used in clinical practice, particularly in patients with diabetes mellitus and potential response to statin therapy.4,9–11 Elevated levels have also been associated with altered cardiac structure and function, as well as adverse long-term consequences.12 In addition, hsCRP has been suggested to play a role in atherosclerosis and its complications, although genetic studies suggest that the association with adverse outcomes may not be causal.13,14 In contrast, MPO has been shown to directly promote the catalytic consumption of nitric oxide, leading to the development of endothelial dysfunction.15–17 MPO is a leukocyte-derived hemoprotein that has been linked in the development and subsequent instability of atherosclerotic plaques.18,19 Previous studies have shown MPO to have prognostic significance in subjects with unstable angina, as well as after acute myocardial infarction, acute heart failure, and chronic stable heart failure, as well as healthy middle-aged and elderly subjects.3,18,20–23 Recently, it was also found that MPO remained a statistically significant prognostic indicator of cardiovascular risk in a large stable CAD population.8 In contrast, the natriuretic peptide family is a group of endogenous peptides primarily produced in the heart that provide counter-regulatory effects on a wide range of organs to maintain perfusion and reduce overloading status of the vasculature.24 BNP has recently been shown to be elevated in acute coronary syndromes without necessarily having myocardial infarction, and it may reflect not only the underlying impairment of left ventricular function but also the severity of the ischemic episode.25 Altogether, this combination of biomarkers offers complementary mechanistic insights during cardiac evaluation in stable patients, although only a small subset of patients demonstrated positive results for all 3 biomarkers in our relatively stable patient cohort.
Our study further explored the impact of glycemic control on the prognostic value of cardiac biomarkers, particularly as the latest guidelines have highlighted a subset of “at-risk” patients that is thought to have heightened risk for developing diabetes mellitus and future cardiovascular risk.7,26 We observed a graded increase in the levels of each of our corresponding biomarkers as patients were determined to have nondiabetes, prediabetes, and diabetes. The overall trend toward increased risk for MACEs was similar among all groups on the basis of their CBS. Similarly, the CBS provided significant prognostic value among subjects for whom no significant angiographic evidence of stenosis was discovered, who are most often considered to have lower risk.
The strength of this study is the considerable size of the patient population. This contemporary cohort of stable cardiac patients is representative of current clinical practice. The focus on the homogenous elective coronary angiography population and the availability and inclusion of only biomarkers cleared by the United States Food and Drug Administration for the analyses strengthen the study, as the present biomarkers, although clinically available for use, are not routinely measured. Including them in the analysis provides insight into a nonacute, troponin-negative population, which has yet to be thoroughly investigated. Incremental contributions of these cardiac biomarkers toward risk stratification above and beyond standard clinical and biochemical characteristics in this population have also not been thoroughly tested, particularly with rigorous statistical evaluation or covariate adjustments. Potential weaknesses of the study population arise because a clinical trial cohort of patients who underwent coronary angiography was used, and thus resulting in, particularly because they were already undergoing cardiac evaluation. Moreover, our data relate to prognostic rather than diagnostic applications of these biomarkers. We also did not have high-sensitivity troponin assays in this cohort, which was deemed troponin negative by currently approved troponin assays. Last, although our results were based on previously used cut points, they may overestimate the strengths of the risk relations. With different studies using different cutoff values for different populations, there is a need to identify clinically useful cut points on the basis of consensus of results.
Acknowledgments
This research was supported in part by Grants P01HL087018-020001, P01HL076491-055328, 1R01HL103866, and 1RO1HL103931 from the National Institutes of Health, Bethesda, Maryland; Grant 1UL1RR024989 from the Cleveland Clinic Clinical Research Unit of the Case Western Reserve University CTSA, Cleveland, Ohio; and the LeDucq Foundation, Paris, France. Dr. Hazen is also supported in part by a gift from the Leonard Kreiger Fund, Cleveland, Ohio. Supplies and partial funding for performance of fasting lipid profiles, glucose, creatinine, apolipoprotein A1, apolipoprotein B, high-sensitivity C-reactive protein, myeloperoxidase, and B-type natriuretic peptide used in this study were provided by Abbott Laboratories, Inc., Abbott Park, Illinois.
Footnotes
Disclosures
Dr. Tang received research grant support from Abbott Laboratories, Abbott Park, Illinois. Dr. Hazen is a co-inventor on pending and issued patents held by the Cleveland Clinic relating to cardiovascular diagnostics. Dr. Hazen has received consulting fees from AstraZeneca Pharmaceuticals, Wilmington, Delaware; Cleveland HeartLab, Cleveland, Ohio; Eli Lilly & Company, Indianapolis, Indiana; Esperion Therapeutics, Plymouth, Michigan; LipoScience Inc., Raleigh, North Carolina; Merck & Company, Whitehouse Station, New Jersey, and Pfizer, Inc., New York, New York. Dr. Hazen has received research funding from Abbott Laboratories, Cleveland HeartLab, Esperion Therapeutics, and LipoScience Inc. Dr. Hazen has the right to receive royalty payments for inventions or discoveries related to cardiovascular diagnostics from Abbott Laboratories; Cleveland HeartLab; Frantz BioMarkers, LLC, Mentor, Ohio; LipoScience Inc.; and Siemens Healthcare, Erlangen, Germany.
References
- 1.Sabatine MS, Morrow DA, de Lemos JA, Gibson CM, Murphy SA, Rifai N, McCabe C, Antman EM, Cannon CP, Braunwald E. Multi-marker approach to risk stratification in non-ST elevation acute coronary syndromes: simultaneous assessment of troponin I, C-reactive protein, and B-type natriuretic peptide. Circulation. 2002;105:1760–1763. doi: 10.1161/01.cir.0000015464.18023.0a. [DOI] [PubMed] [Google Scholar]
- 2.de Lemos JA, Morrow DA, Bentley JH, Omland T, Sabatine MS, McCabe CH, Hall C, Cannon CP, Braunwald E. The prognostic value of B-type natriuretic peptide in patients with acute coronary syndromes. N Engl J Med. 2001;345:1014–1021. doi: 10.1056/NEJMoa011053. [DOI] [PubMed] [Google Scholar]
- 3.Brennan ML, Penn MS, VanLente F, Nambi V, Shishehbor MH, Aviles RJ, Goormastic M, Pepoy ML, McErlean ES, Topol EJ, Nissen SE, Hazen SL. Prognostic value of myeloperoxidase in patients with chest pain. N Engl J Med. 2003;349:1595–1604. doi: 10.1056/NEJMoa035003. [DOI] [PubMed] [Google Scholar]
- 4.Ridker PM, Hennekens CH, Buring JE, Rifai N. C-reactive protein and other markers of inflammation in the prediction of cardiovascular disease in women. N Engl J Med. 2000;342:836–843. doi: 10.1056/NEJM200003233421202. [DOI] [PubMed] [Google Scholar]
- 5.Morrow DA, Braunwald E. Future of biomarkers in acute coronary syndromes: moving toward a multimarker strategy. Circulation. 2003;108:250–252. doi: 10.1161/01.CIR.0000078080.37974.D2. [DOI] [PubMed] [Google Scholar]
- 6.Executive summary of the third report of the National Cholesterol Education Program (NCEP) Expert Panel on Detection, Evaluation, and Treatment of High Blood Cholesterol in Adults (Adult Treatment Panel III) JAMA. 2001;285:2486–2497. doi: 10.1001/jama.285.19.2486. [DOI] [PubMed] [Google Scholar]
- 7.Standards of medical care in diabetes—2012. Diabetes Care. 2012;35(suppl):S11–S63. doi: 10.2337/dc12-s011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Tang WH, Wu Y, Nicholls SJ, Hazen SL. Plasma myeloperoxidase predicts incident cardiovascular risks in stable patients undergoingmedical management for coronary artery disease. Clin Chem. 2011;57:33–39. doi: 10.1373/clinchem.2010.152827. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Ridker PM, Danielson E, Fonseca FA, Genest J, Gotto AM, Jr, Kastelein JJ, Koenig W, Libby P, Lorenzatti AJ, MacFadyen JG, Nordestgaard BG, Shepherd J, Willerson JT, Glynn RJ. Rosuvastatin to prevent vascular events in men and women with elevated C-reactive protein. N Engl J Med. 2008;359:2195–2207. doi: 10.1056/NEJMoa0807646. [DOI] [PubMed] [Google Scholar]
- 10.Ridker PM, Rifai N, Clearfield M, Downs JR, Weis SE, Miles JS, Gotto AM Jr. Measurement of C-reactive protein for the targeting of statin therapy in the primary prevention of acute coronary events. N Engl J Med. 2001;344:1959–1965. doi: 10.1056/NEJM200106283442601. [DOI] [PubMed] [Google Scholar]
- 11.Ridker PM, Rifai N, Rose L, Buring JE, Cook NR. Comparison of C-reactive protein and low-density lipoprotein cholesterol levels in the prediction of first cardiovascular events. N Engl J Med. 2002;347:1557–1565. doi: 10.1056/NEJMoa021993. [DOI] [PubMed] [Google Scholar]
- 12.Tang WH, Shrestha K, Van Lente F, Troughton RW, Martin MG, Borowski AG, Jasper S, Klein AL. Usefulness of C-reactive protein and left ventricular diastolic performance for prognosis in patients with left ventricular systolic heart failure. Am J Cardiol. 2008;101:370–373. doi: 10.1016/j.amjcard.2007.08.038. [DOI] [PubMed] [Google Scholar]
- 13.Anand SS, Yusuf S. C-reactive protein is a bystander of cardiovascular disease. Eur Heart J. 2010;31:2092–2096. doi: 10.1093/eurheartj/ehq242. [DOI] [PubMed] [Google Scholar]
- 14.Elliott P, Chambers JC, Zhang W, Clarke R, Hopewell JC, Peden JF, Erdmann J, Braund P, Engert JC, Bennett D, Coin L, Ashby D, Tzoulaki I, Brown IJ, Mt-Isa S, McCarthy MI, Peltonen L, Freimer NB, Farrall M, Ruokonen A, Hamsten A, Lim N, Froguel P, Waterworth DM, Vollenweider P, Waeber G, Jarvelin MR, Mooser V, Scott J, Hall AS, Schunkert H, Anand SS, Collins R, Samani NJ, Watkins H, Kooner JS. Genetic loci associated with C-reactive protein levels and risk of coronary heart disease. JAMA. 2009;302:37–48. doi: 10.1001/jama.2009.954. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Hazen SL, Zhang R, Shen Z, Wu W, Podrez EA, MacPherson JC, Schmitt D, Mitra SN, Mukhopadhyay C, Chen Y, Cohen PA, Hoff HF, Abu-Soud HM. Formation of nitric oxide-derived oxidants by myeloperoxidase in monocytes: pathways for monocyte-mediated protein nitration and lipid peroxidation In vivo. Circ Res. 1999;85:950–958. doi: 10.1161/01.res.85.10.950. [DOI] [PubMed] [Google Scholar]
- 16.Schmitt D, Shen Z, Zhang R, Colles SM, Wu W, Salomon RG, Chen Y, Chisolm GM, Hazen SL. Leukocytes utilize myeloperoxidase-generated nitrating intermediates as physiological catalysts for the generation of biologically active oxidized lipids and sterols in serum. Biochemistry. 1999;38:16904–16915. doi: 10.1021/bi991623w. [DOI] [PubMed] [Google Scholar]
- 17.Vita JA, Brennan ML, Gokce N, Mann SA, Goormastic M, Shishehbor MH, Penn MS, Keaney JF, Jr, Hazen SL. Serum myeloperoxidase levels independently predict endothelial dysfunction in humans. Circulation. 2004;110:1134–1139. doi: 10.1161/01.CIR.0000140262.20831.8F. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Nicholls SJ, Hazen SL. Myeloperoxidase and cardiovascular disease. Arterioscler Thromb Vasc Biol. 2005;25:1102–1111. doi: 10.1161/01.ATV.0000163262.83456.6d. [DOI] [PubMed] [Google Scholar]
- 19.Nicholls SJ, Hazen SL. Myeloperoxidase, modified lipoproteins, and atherogenesis. J Lipid Res. 2009;50(suppl):S346–S351. doi: 10.1194/jlr.R800086-JLR200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Tang WH, Shrestha K, Troughton RW, Borowski AG, Klein AL. Integrating plasma high-sensitivity C-reactive protein and myeloperoxidase for risk prediction in chronic systolic heart failure. Congest Heart Fail. 2011;17:105–109. doi: 10.1111/j.1751-7133.2011.00221.x. [DOI] [PubMed] [Google Scholar]
- 21.Nicholls SJ, Tang WH, Brennan D, Brennan ML, Mann S, Nissen SE, Hazen SL. Risk prediction with serial myeloperoxidase monitoring in patients with acute chest pain. Clin Chem. 2011;57:1762–1770. doi: 10.1373/clinchem.2011.166827. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Reichlin T, Socrates T, Egli P, Potocki M, Breidthardt T, Arenja N, Meissner J, Noveanu M, Reiter M, Twerenbold R, Schaub N, Buser A, Mueller C. Use of myeloperoxidase for risk stratification in acute heart failure. Clin Chem. 2010;56:944–951. doi: 10.1373/clinchem.2009.142257. [DOI] [PubMed] [Google Scholar]
- 23.Naruko T, Furukawa A, Yunoki K, Komatsu R, Nakagawa M, Matsumura Y, Shirai N, Sugioka K, Takagi M, Hozumi T, Itoh A, Haze K, Yoshiyama M, Becker AE, Ueda M. Increased expression and plasma levels of myeloperoxidase are closely related to the presence of angiographically-detected complex lesion morphology in unstable angina. Heart. 2010;96:1716–1722. doi: 10.1136/hrt.2009.187609. [DOI] [PubMed] [Google Scholar]
- 24.Tang WH. B-type natriuretic peptide: a critical review. Congest Heart Fail. 2007;13:48–52. doi: 10.1111/j.1527-5299.2007.05622.x. [DOI] [PubMed] [Google Scholar]
- 25.Fonarow GC, Peacock WF, Phillips CO, Givertz MM, Lopatin M ADHERE Scientific Advisory Committee and Investigators. Admission B-type natriuretic peptide levels and in-hospital mortality in acute decompensated heart failure. J Am Coll Cardiol. 2007;49:1943–1950. doi: 10.1016/j.jacc.2007.02.037. [DOI] [PubMed] [Google Scholar]
- 26.Tabak AG, Herder C, Rathmann W, Brunner EJ, Kivimaki M. Prediabetes: a high-risk state for diabetes development. Lancet. 2012;379:2279–2290. doi: 10.1016/S0140-6736(12)60283-9. [DOI] [PMC free article] [PubMed] [Google Scholar]