Scheme 1.
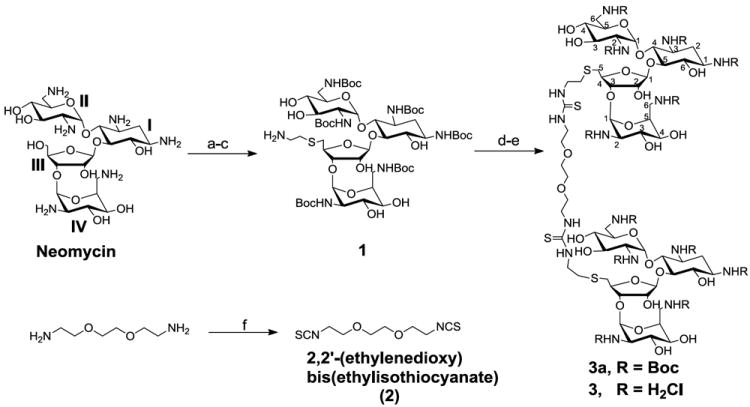
Reagents and conditions: a-e: (a) (Boc)2O, DMF, H2O, Et3N, 60°C, 5 h, 60%. (b) TPS-Cl, pyridine, r.t., 40 h, 50%. (c) HSCH2CH2NH2.HCl, EtOH, EtONa, r.t., 12h, 60%. (d) 2, pyridine, DMAP, r.t., 12 h, 95%. (e) 4 M HCl/dioxane, HSCH2CH2SH, r.t., 5 min, 73%. (f) TCDP, dry DCM, r.t., 4 h, 94%.
