Figure 3.
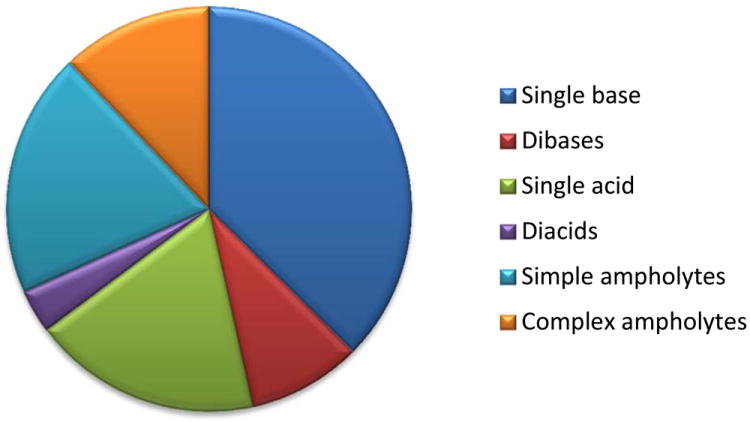
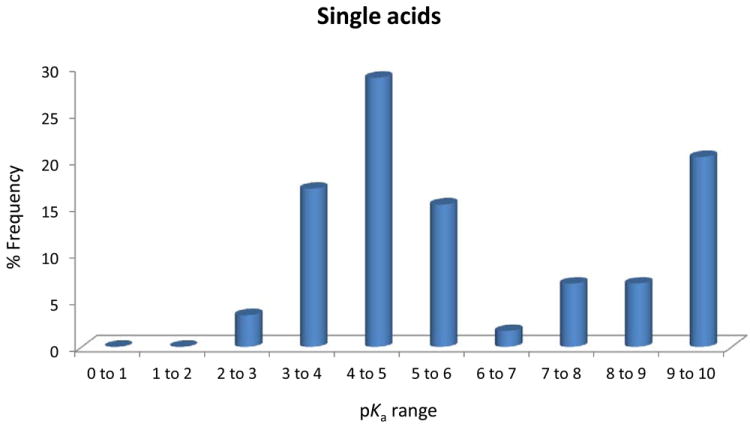
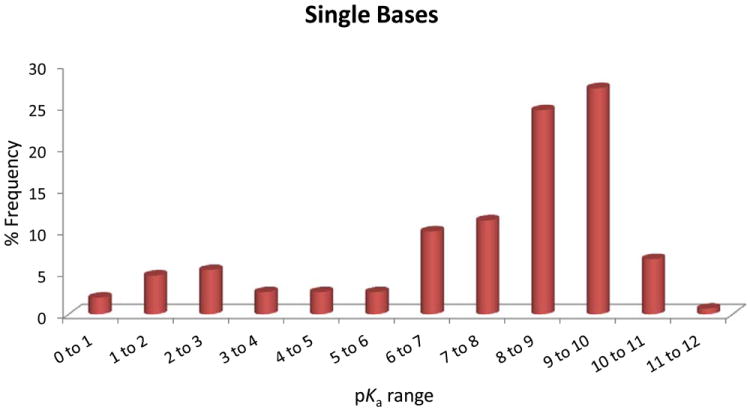
(A), Proportion of compound categories for ionisable oral drugs.7 (B), pKa distributions of oral drugs containing a single acidic functional group, or (C) single basic functional group. Compounds with the following criteria were classified as always ionised: acids with pKa values < 0 and bases with pKa values > 12, plus compounds with permanently charged groups (e.g., quaternary nitrogen atoms). Acids with pKa values above 10 or bases with pKa values below 0.0 were considered neutral. The remaining ionisable compounds (acid pKa range 0-10 and base pKa range 0-12) were divided into the following groups: single acid-containing substances, single base-containing substances, compounds with two acidic groups, compounds with two basic groups, simple ampholytes (one acidic and one basic group) and other complex combinations of acidic and basic groups (complex ampholytes). pKa values were binned into single log unit ranges (i.e. 0.0 < X ≤ 1.0, 1.0 < X ≤ 2.0, etc.). The histogram column heights are expressed as a percentage.
