Abstract
Objective
Previous research has suggested that childhood emotional abuse, physical abuse, and sexual abuse are associated with an increased risk for ischemic heart disease. Our objective was to examine whether childhood abuse predicted incident metabolic syndrome, a precursor to heart disease, in mid-life women.
Methods
Participants were 342 (114 Black, 228 White) women from the Pittsburgh site of the Study of Women’s Health Across the Nation (SWAN). SWAN included a baseline assessment of premenopausal or early perimenopausal women in midlife (mean age = 45.7), and women were evaluated for presence of the metabolic syndrome over 7 annual follow-up visits. Women were classified as having metabolic syndrome if they met 3 of the following criteria: waist circumference > 88 cm, triglycerides ≥ 150 mg/dl, HDL < 50 mg/dl, SBP ≥ 130 or DBP ≥ 85 mmHg or on blood pressure medication, and fasting glucose ≥ 110 mg/dl or diabetic. The Childhood Trauma Questionnaire is a standardized measure that retrospectively assesses three domains of abuse in childhood and adolescence: emotional, physical, and sexual abuse.
Results
Approximately 34% of the participants reported a history of abuse. Cox model survival analysis showed that physical abuse was associated with incident metabolic syndrome over the course of seven years (HR = 2.12, p = .02), adjusted for ethnicity, age at baseline, and time-dependent menopausal status. Sexual abuse and emotional abuse were unrelated to the metabolic syndrome.
Conclusion
This is the first study to show that a history of childhood abuse, specifically physical abuse, is related to the development of metabolic syndrome in mid-life women.
Keywords: childhood abuse, metabolic syndrome, menopause
Introduction
Epidemiological studies of childhood abuse show that as many as a quarter of all women report a history of childhood abuse (Felitti et al., 1998; Walker et al., 1999). One such study assessed over 9000 women from a health maintenance organization and found that 27% of women reported physical abuse, 25% reported sexual abuse, and 13% reported emotional abuse (Dube et al., 2001). Childhood abuse is commonly associated with negative psychological health problems, including depression, post-traumatic stress disorder, and alcohol and substance abuse (Brewin, Andrews, & Valentine, 2000; Simpson & Miller, 2002; Widom, Dumont, & Czaja, 2007). A recent meta-analysis suggested that childhood abuse also predicts physical health problems, and the magnitude of this relationship is similar to the association between abuse and psychological outcomes (Wegman & Stetler, 2009). For example, one study found that emotional, physical and sexual abuse significantly increased the risk of ischemic heart disease by 1.4 to 1.7-fold compared to people with no history of any type of abuse (Dong, Anda, et al., 2004). It is possible that childhood abuse not only predicts cardiac events, but that abuse is also associated with indicators of cardiometabolic risk prior to the occurrence of clinical events.
An important pre-clinical syndrome is the clustering of metabolic risk factors including central adiposity, hypertension, dyslipidemia, and insulin resistance. The metabolic syndrome predicts the development of atherosclerosis, myocardial infarction, and type 2 diabetes (Grundy et al., 2005; Ninomiya et al., 2004; Smith, 2007). Women may be particularly vulnerable. A recent study from the Third National Health and Nutrition Examination Survey (NHANES) reported that the metabolic syndrome was an independent risk factor for all-cause mortality and cardiovascular-related mortality in postmenopausal women, although not in premenopausal women or men (Lin, Caffrey, Chang, & Lin, 2010). Furthermore, as women age (≥ 55 years old), the metabolic syndrome becomes a stronger predictor of cardiovascular disease compared to when they are younger (Lorenzo, Williams, Hunt, & Haffner, 2007).
To our knowledge only one study has examined whether a history of childhood abuse or neglect is associated with aspects of the metabolic syndrome in adulthood. In men and women enrolled in the Dunedin New Zealand Study, a likely history of childhood abuse or neglect was associated with a 1.39 odds ratio for having a clustering of metabolic risk biomarkers (at least three of the following: overweight, high blood pressure, high total cholesterol, low high-density cholesterol, high glycated hemoglobin, and low VO2max adjusted for body weight) at age 32 (Danese, Pariante, Caspi, Taylor, & Poulton, 2007). The current study aims to test the association between childhood abuse and incident metabolic syndrome defined by the National Cholesterol Education Program Adult Treatment Panel III criteria in women during mid-life aging, a time of increased metabolic syndrome prevalence. In previous literature, physical abuse and sexual abuse have predicted obesity in the same sample of mid-life women (Midei, Matthews, & Bromberger, 2010). Thus, we hypothesized that childhood abuse, specifically physical abuse and sexual abuse, would be associated with incident metabolic syndrome in mid-life, adjusting for ethnicity, age at baseline, and time-dependent menopausal status. Due to our previous report (Midei et al., 2010) that abuse predicted central adiposity in this sample, we also conducted sub-analyses to examine whether components of the metabolic syndrome other than central adiposity, were driving any overall associations. Significant results would suggest that the list of psychosocial stressors predicting the metabolic syndrome, such as depression and low socioeconomic status (Goldbacher & Matthews, 2007; Matthews, 2008), may also include childhood abuse. Identifying susceptible subgroups at high risk for the metabolic syndrome may help with prevention and intervention for cardiovascular-related morbidity and mortality.
Method
Participants
The present study was conducted using data from participants in the Study of Women’s Health Across the Nation (SWAN), a multi-site, community-based, cohort investigation of menopause and aging. The sample included participants that enrolled in the Mental Health Study at the Pittsburgh SWAN site. The Mental Health study collected detailed mental health data at baseline and conducted annual follow-up visits. Participants were eligible for inclusion in SWAN if they were 42 to 52 years old, had at least one menstrual period in the past three months, did not use oral contraceptives or other female reproductive hormones, had not undergone a hysterectomy or bilateral oophorectomy, and not pregnant or breast-feeding. All instruments and study protocol were approved by the University of Pittsburgh Institutional Review Board, and written informed consent was obtained from all participants.
Of the 463 Pittsburgh SWAN participants eligible for the Mental Health Study, 96% enrolled (n = 443). The Mental Health Study retention rate was approximately 82% through follow-up visit 9 (n = 365). Twenty-three participants did not complete the Childhood Trauma Questionnaire (CTQ), which was administered at visit 8 or 9. The final sample used in this study included 342 women (113 Black, 229 White).
Procedure
SWAN and Mental Health Study baseline assessments were conducted in 1996 and 1997. SWAN participants completed self-administered and interviewer-administered questionnaires and a physical examination at the SWAN baseline and annually (three months) thereafter. Core SWAN data collection provided metabolic syndrome data at baseline and selected visits (visits 1, 3, 4, 5, 6, 7) due to the cost of assays. The Mental Health Study provided childhood abuse data (measured by the CTQ) from visit 8.
Measures
Childhood abuse
Childhood abuse was assessed using the 28-item short form of the Childhood Trauma Questionnaire (CTQ) (Bernstein & Fink, 1998), a self-report instrument that assesses emotional abuse (5 items), physical abuse (5 items), and sexual abuse (5 items). The CTQ also measures neglect and minimization/denial subscales, although these scales were not used in the present analyses. Subjects rated statements about childhood experiences on five-point Likert-type scales (“never true” to “very often true”). Most items were phrased in objective and behavioral terms (e.g., “When I was growing up, someone touched me in a sexual way or made me touch them”), whereas some items required subjective evaluation (e.g., “When I was growing up, I believe I was sexually abused”). Items were summed to yield scores on three subscales (emotional abuse, physical abuse, and sexual abuse) with scores ranging from 5 to 25. Clinical cut-off points have been validated and have sensitivity and specificity at 0.85 or higher relative to clinical interview (therapists’ ratings of childhood maltreatment) (Bernstein & Fink, 1998; Bernstein et al., 2003; Walker et al., 1999). Scores for each scale that were at or above these thresholds were classified as positive for abuse: emotional abuse = 10, physical abuse = 8, and sexual abuse = 8. Women scoring at or above the clinical threshold on any one subscale were classified as having been exposed to any abuse. If scoring below all clinical cut-off points, an individual was classified as not exposed to abuse. The CTQ has strong test-retest reliability and convergent validity with clinical interview and therapist ratings (Bernstein et al., 1994; Bernstein et al., 2003; Walker et al., 1999). Responses from SWAN participants showed that the CTQ had strong internal consistency, Cronbach’s α = 0.80–0.94 for the subscales in this investigation.
Classification of metabolic syndrome
We defined the metabolic syndrome using the National Cholesterol Education Program Adult Treatment Panel III definition (Grundy et al., 2005). Women were identified as having the metabolic syndrome if they met three of the following five criteria: waist circumference (WC) ≥ 88 cm, triglycerides ≥ 150 mg/dl, high-density lipoprotein cholesterol (HDL) < 50 mg/dl, systolic blood pressure (SBP) ≥ 130 or diastolic blood pressure (DBP) ≥ 85 mmHg or on blood pressure medication, and fasting glucose ≥ 110 mg/dl.
WC was measured in centimeters at the level of the natural waist, defined as the narrowest part of the torso as seen from the anterior aspect. In cases where a waist narrowing was difficult to identify, the measurement was taken at the smallest horizontal circumference in the area between the ribs and the iliac crest. Standardized protocols were used by trained personnel who were certified by the coordinating center to measure WC.
A fasting blood draw was used to assess lipids and glucose and was targeted to days 2–5 of the follicular phase of the menstrual cycle in menstruating women and within 90 days of the anniversary of the baseline examination date. If a timed sample could not be obtained after two attempts, or if a participant ceased menstruating, a random fasting sample was taken within a 90-day window of the annual visit. Three vacutainers (Becton Dickinson; Franklin Lakes, NJ) of blood were collected for cardiovascular measures: a 10mL red-top (serum) tube, a 5mL blue-top (citrate plasma) tube, and a 10mL lavender-top (EDTA-plasma) tube. All specimen collection packets were provided by the University of Michigan Central Laboratory (Ann Arbor, MI). Glucose was assayed from the red top tube, and lipids were assayed from the lavender top tube.
The red-top tube was inverted 5 times, stored for 30 minutes at room temperature, refrigerated at 4°C for 30–90 minutes and then centrifuged at 2000 rpm for 20 minutes. The serum was aliquotted, frozen at −20°C, and shipped on dry ice to the central laboratory in less than 30 days. The lavender-top tube was inverted 8–10 times, placed on blue ice or refrigerated at 4°C for up to 2 hours and then centrifuged at 2000 rpm for 20 minutes. The plasma was aliquotted, refrigerated at 4°C, and shipped on blue ice to the central laboratory in 7 days or less. The central laboratory was the Medical Research Laboratories (Highland Heights, KY) and remained certified by the National Heart Lung and Blood Institute, Centers for Disease Control Part III program.
Glucose was measured using a hexokinase-coupled reaction (Boehringer Mannheim Diagnostics, Indianapolis, IN). Total cholesterol and triglyceride levels were analyzed by enzymatic methods on a Hitachi 747 analyzer. HDL cholesterol was isolated using heparin-2M manganese chloride.
Blood pressure was measured according to a standardized laboratory protocol, with readings taken on the right arm, with the respondent seated and feet flat on the floor for at least five minutes prior to measurement. Respondents had not smoked or consumed any caffeinated beverage within 30 minutes of blood pressure measurement. Appropriate cuff size was determined based on arm circumference. A standard mercury sphygmomanometer was used to record systolic and diastolic pressures at the first and fifth phase Korotkoff sounds. Two sequential blood pressure values were obtained within a five-minute interval, and the average blood pressure was used in analyses.
Traditional risk factors
Cigarette smoking was assessed by the item, “Since your last study visit, have you smoked cigarettes regularly (at least one cigarette a day)?” A positive response to this item indicated a current smoker. Smoking status was measured at baseline and each follow-up visit; however, there was a high degree of agreement between baseline status and each visit status (kappa statistics ranged from 0.78 to 0.90) so we used baseline smoking status in analytic models.
A physical activity index was created from the Kaiser Physical Activity Survey, which was originally adapted from the Baecke physical activity questionnaire (Baecke, Burema, & Frijters, 1982; Sternfeld, Ainsworth, & Quesenberry, 1999). The survey assessed physical activity in three domains over the past year: 1) household and care giving, 2) sports and exercise, and 3) work. The physical activity index was a summary score of two domains, household/care giving and sports/exercise, and ranged from 3–12, with high scores reflecting greater overall physical activity. The work domain was not included in the summary score because many women were not employed. Previous work has shown that this survey has strong test-retest reliability and validity in White and Black women (Ainsworth, Sternfeld, Richardson, & Jackson, 2000; Sternfeld et al., 1999). Analyses used the physical activity index measured at baseline; after baseline the full scale at each visit was not administered.
To identify alcohol abuse, we assessed diagnosis of lifetime alcohol abuse and dependence with the Structured Clinical Interview for DSM Disorders (SCID) (Spitzer, William, Gibbon, & First, 1992). The SCID is a standard semi-structured interview used in many clinical and epidemiological studies to assess psychiatric disorders. It was conducted by a trained interviewer at baseline in SWAN.
Depressive symptom levels were assessed by the Center for Epidemiological Studies Depression scale (CES-D) (Radloff, 1977). This is a 20-item scale measuring depressive symptoms with four-level responses indicating frequency of experiencing each symptom in the past week. Scores ranged from 0 to 60 and are dichotomized with those scoring at or above 16 being classified as potentially clinically depressed. The CES-D has well-established reliability (Cronbach’s alpha=.85) and has been shown to correlate well with other depressive symptom questionnaires and with interview assessments of severity of depression (Fechner-Bates, Coyne, & Schwenk, 1994). The CES-D was given at baseline and all follow-up visits. There was moderate agreement between depression scores across visits (intraclass correlation = 0.50); suggesting that depression status changed over time. Repeated measures of depression were included in analyses in order to account for the possibility that a participant may endorse depressive symptoms at one visit but not the following visit. This was important to do because depressive symptoms have been associated with both childhood abuse and the metabolic syndrome, making it necessary to adjust for these symptoms at each visit. A dichotomous time-dependent depression status variable was created for each visit or time point, with CES-D scores < 16 coded as 0 and scores ≥ 16 coded as 1.
Childhood SES was measured by the highest level of either parent’s education, assessed at visit 7. Adulthood SES was measured by the highest year of schooling completed by the participant, assessed at baseline.
Covariates
Age, ethnicity, and time-dependent menopausal status were the covariates used in all models. Participants self-identified as Caucasian or African American during the baseline SWAN interview. Menopause status was determined from self-reported bleeding patterns over the year preceding the visit categorized as follows: bleeding in the previous 3 months with no change in cycle predictability in the past year was considered pre-menopausal, bleeding in the previous 3 months with a decrease in cycle predictability in the past year was considered early peri-menopausal, fewer than 12 and more than 3 months of amenorrhea was considered late peri-menopausal, and 12 months or more of amenorrhea was considered post-menopausal. Menopausal status was another variable (in addition to depression) that changed over time as women progressed through the menopausal transition. That is, a woman may have been premenopausal at the start of the study, with her final menstrual period occurring later in the study. Furthermore, the timing of the transition varied between participants. We accounted for this time-dependent variable, menopausal status, by including status at each visit in the analyses. Specifically, for each visit or time point, a categorical variable was created with pre-menopause coded as 0, early peri-menopause coded as 1, peri-menopause coded as 2, late peri-menopause coded as 3, post-menopause/surgical menopause coded as 4, and unknown coded as 5.
Statistical Analyses
Differences between participants who completed the CTQ and participants who did not complete the CTQ were reported previously (Midei et al., 2010). Chi-square analyses were used to examine ethnicity differences for abuse history. Logistic regressions were used to examine whether history of any abuse (each subtype entered in separate equations) was associated with the presence of the metabolic syndrome at baseline, controlling for ethnicity, age at baseline, and menopausal status at baseline. Logistic regression provides an odds ratio, or the likelihood of developing the metabolic syndrome based on the history of childhood abuse. Potential risk factors for the metabolic syndrome, such as cigarette smoking, alcohol abuse, physical activity, childhood SES, and adulthood SES, were entered as a step in the model if significant relationships were found between abuse and baseline metabolic syndrome.
Among those without metabolic syndrome at baseline, Cox proportional hazards modeling was used to examine whether history of any abuse (each subtype entered in separate equations) predicted incident metabolic syndrome over the follow-up annual visits. The time variable was time from baseline to the annual visit when the first episode of metabolic syndrome occurred. Cox model provides a hazard ratio statistic, and interpretation is similar to an odds ratio. Analyses first controlled for ethnicity, age at baseline, and time-dependent menopausal status. If significant relationships were found between abuse and incident metabolic syndrome during the menopausal transition, traditional risk factors for the metabolic syndrome were then entered as a step in the model. Additionally, abuse was also tested as a predictor of the individual components of the metabolic syndrome. Analyses were performed using SAS, version 9.1 (Allison, 2010).
Results
Table 1 shows descriptive characteristics of the study sample.Table 2 shows the number of participants reporting each type of abuse, as well as a breakdown of types of abuse by ethnicity. Thirty-four percent (n = 115) of the sample reported experiencing some form of childhood abuse. Analyses showed that Blacks experienced significantly more physical abuse than did Whites (χ2= 15.49, p < .001).
Table 1.
Sample Characteristics (percent and number of subjects) for the full sample and by race
| Characteristic | % (N) of Total Sample N = 342 |
% (N) of Blacks n = 114 |
% (N) of Whites n = 228 |
|---|---|---|---|
| Age (M) | 45.7 | 45.5 | 45.7 |
| Metabolic syndrome at baseline | 18 (60) | 20 (23) | 16 (37) |
| Current smoker at baselinea | 17 (57) | 22 (25) | 14 (32) |
| Lifetime history of alcohol abuse/dependence | 12 (40) | 15 (17) | 10 (23) |
| Depression at baseline = (CES-D ≥ 16)b | 25 (84) | 31 (35) | 22 (49) |
| Childhood SES = (Parent education ≤ HS) | 49 (169) | 50 (57) | 49 (112) |
| Adulthood SES = (Subject education ≤ HS) | 24 (82) | 26 (30) | 23 (52) |
| Incident metabolic syndrome, visit 1 – 7c | 21 (59) | 25 (23) | 19 (36) |
CES-D = Center for Epidemiological Studies Depression; SES = socioeconomic status HS = high school
More blacks reported being current smokers than whites (χ2 = 3.70, p = .06).
More blacks reported being depressed than whites (χ2 = 3.48, p = .06).
Incident metabolic syndrome was calculated for women free of metabolic syndrome at baseline (342 – 60 = 282). Thus, out of 282 women, 59 women (or 21% of the remaining sample), were classified as having incident metabolic syndrome during the follow-up visits.
Table 2.
Rates of Reported Abuse in the Full Sample (N = 342)
| Type of Abuse/Neglect | % (N) of Total Sample | % (N) of Blacks | % (N) of Whites |
|---|---|---|---|
| Emotional abuse | 21 (71) | 18 (20) | 22 (51) |
| Physical abusea | 17 (59) | 26 (30) | 13 (29) |
| Sexual abuse | 15 (51) | 17 (19) | 14 (32) |
| Any abuse | 34 (115) | 38 (43) | 32 (72) |
More blacks reported physical abuse than whites (χ2 = 9.72, p < .01).
Logistic analyses showed that childhood abuse did not predict the presence of the metabolic syndrome at baseline while controlling for age, ethnicity and menopausal status at baseline: emotional abuse (OR = 1.10, 95% CI 0.55 – 2.19, p = .79), physical abuse (OR = 0.98, 95% CI 0.45 – 2.13, p = .96), and sexual abuse (OR = 1.72, 95% CI 0.82 – 3.59, p = .15). Furthermore, participants with any type of abuse (emotional or physical or sexual) did not have higher risk for metabolic syndrome at baseline (OR = 1.22, 95% CI 0.67 – 2.20, p = .52).
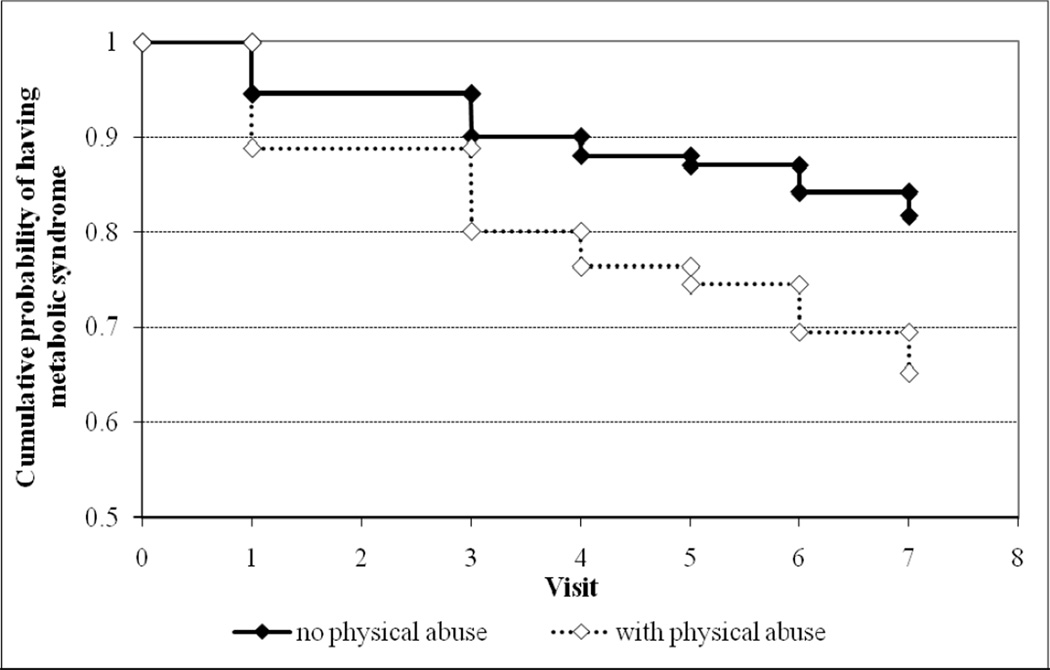
In the 282 women free of metabolic syndrome at baseline, 59 women were identified with the metabolic syndrome over the follow-up visits. Cox proportional hazards modeling showed that physical abuse was associated with incident metabolic syndrome (HR = 2.12, 95% CI 1.15 – 3.91, p = .02), controlling for age at baseline, ethnicity, and time-dependent menopausal status. Figure 1 displays the relationship between childhood physical abuse and incident metabolic syndrome with a survival curve. Emotional abuse and sexual abuse were not associated with incident metabolic syndrome, (HR = 1.37, 95% CI 0.75 – 2.53, p = .31) and (HR = 1.29, 95% CI 0.63 – 2.62, p = .49), respectively. Physical abuse continued to be associated with incident metabolic syndrome even when further controlling for cigarette smoking, physical activity, alcohol abuse, depressive symptoms, childhood SES, and adulthood SES (HR = 2.02, 95% CI 1.02 – 4.02, p = .04).
Figure 1.
Cumulative probability of surviving the 7 follow-up visits without a diagnosis of the metabolic syndrome, controlling for ethnicity, age at baseline, and menopausal status at baseline.
Blacks and whites in our sample reported significantly different rates of physical abuse; thus, we tested whether the relationship between physical abuse and incident metabolic syndrome varied by ethnicity. We created an interaction term (physical abuse*race) and added the term as an independent variable in the Cox regression analysis. The interaction term did not significantly predict incident metabolic syndrome (p = .61), suggesting that the relationship between physical abuse and incident metabolic syndrome did not vary by ethnicity.
We also examined whether physical abuse was associated with the incidence (visit 1–7) of elevated risk for each individual component of the metabolic syndrome in individuals without the elevated component at baseline.Table 3 lists the metabolic syndrome components and the number of individuals exceeding the metabolic syndrome threshold for each component, both at the baseline visit and for incidence during visit 1 through visit 7. Cox models showed that physical abuse was associated with the incidence of high waist circumference (≥ 88 cm) (HR = 2.07, 95% CI 1.04 – 4.13, p = .04) and of high fasting glucose (≥ 110 mg/dL) (HR = 2.92, 95% CI 1.45 – 5.86, p < .01) while adjusting for age, ethnicity and menopausal status. Physical abuse was not associated with high BP or medication use, triglycerides, or low HDL (ps > .50).
Table 3.
Descriptive statistics of the metabolic syndrome components at baseline and during follow-up visits
| Metabolic syndrome components |
Number of participants with elevated risk for each component |
Means and standard deviations of components in full sample |
||
|---|---|---|---|---|
| At baseline in full sample N = 342 |
Incident risk for Visit 1 – Visit 7a |
At baseline | At last available visit | |
| WC > 88 cm | 147 | 62 | 88.0 (15.7) | 92.2 (16.9) |
| Glucose ≥ 110 | 23 | 40 | 94.6 (30.2) | 90.9 (22.0) |
| Triglycerides ≥ 150 mg/dl | 53 | 93 | 106.0 (59.4) | 124.3 (68.2) |
| HDL < 50 mg/dl | 125 | 56 | 54.6 (12.5) | 57.8 (14.5) |
| SBP ≥ 130 mmHg or | 76 | 69 | SBP = 115.3 (17.0) | SBP = 116.2 (16.9) |
| DBP ≥ 85 mmHg | DBP = 73.1 (10.2) | DBP = 73.4 (9.4) | ||
| BP meds | 38 | 51 | - | - |
Data listed for participants without the elevated risk component at baseline. For example, for WC, 147 women had elevated risk at baseline, so incident risk (during visits 1–7) for that component was assessed for the remaining 195 women.
WC = waist circumference; HDL = high density lipoproteins; SBP = systolic blood pressure; DBP = diastolic blood pressure; BP = blood pressure
Discussion
The present study tested for an association between reports of childhood abuse and adulthood metabolic syndrome in mid-life black and white women. Results showed that women who reported physical abuse had approximately double the risk for being classified with the metabolic syndrome during mid-life aging. The positive association between physical abuse and incident metabolic syndrome persisted above and beyond traditional risk factors for metabolic syndrome, suggesting physical abuse as a unique predictor in women’s cardiovascular health.
Physical abuse, but not sexual abuse, predicted incident metabolic syndrome, partially supporting our hypothesis. It is possible that the perpetrator of the violence may be an important aspect of the abuse and its link to health. Two of the five items assessing physical abuse in the CTQ implicated a family member, compared to zero of the five items assessing sexual abuse. The CTQ does not obtain information on the perpetrator of the sexual abuse. Future studies should consider gathering information regarding the experience of childhood abuse including the perpetrator in order to evaluate what aspects of the abuse are most salient for subsequent health.
The sub-analyses of the present study point to possible mechanisms between abuse and the metabolic syndrome. Physical abuse was related to incident central adiposity and high fasting glucose; central adiposity and insulin resistance are generally considered the underlying risk factors for the metabolic syndrome (Grundy, 2011). Central adiposity and high fasting glucose may be driven by an unhealthy diet; some studies have shown that using food in response to stress or binge eating partially mediated associations between childhood abuse and obesity (Greenfield & Marks, 2009; Rohde et al., 2008). Dysregulation of the hypothalamic-pituitary-adrenal (HPA) axis and cortisol may be another pathway connecting abuse to central adiposity/fasting glucose. Childhood abuse has been shown to be associated with HPA axis hyper-activation (Cicchetti & Rogosch, 2001; Heim et al., 2000), and cortisol promotes maturation of adipose tissue cells and stimulates storing energy as fat in the presence of glucose and insulin (Gregoire, Genart, Hauser, & Remacle, 1991). Finally, negative emotions such as depression and anger have been shown to partially explain the relationship between abuse and central adiposity or ischemic heart disease, although in the present study we controlled for depressive symptoms (Dong, Giles, et al., 2004; Midei et al., 2010).
There are several implications of this study. Children and adolescents are the most likely to be victimized compared to adults of any age (Finkelhor, Ormrod, & Turner, 2007), and these results suggest that abuse in childhood may have long-lasting health consequences continuing into middle-age. Prevention should be the first priority in the public health domain; programs teaching parenting skills to reduce physical violence in the home have been shown to be effective (Lundahl, Nimer, & Parsons, 2006). Detection of children and adolescents with abuse should be the second priority. Communities can take an active role in encouraging victims to report and can also aim to improve interactions with the legal system. Finally, intervention and treatment may offer opportunities to disconnect the association between childhood abuse and health risk. It is possible that clinicians and clinical-researchers may improve the trajectory of mental and physical health outcomes for victims of interpersonal violence.
There are several strengths and limitations to the current study. SWAN assesses women at mid-life, which is a time period with increased health risks. In addition, the CTQ is a well-validated and reliable assessment of emotional, physical, and sexual abuse. Our study was also able to control for lifestyle factors (cigarette smoking, physical activity, and alcohol abuse) and psychosocial factors (socioeconomic status and depression) related to cardiovascular disease in women. Finally, we were able to test for an abuse by ethnicity interaction and found that the relationship between physical abuse and incident metabolic syndrome did not vary by ethnicity. The present study is limited in understanding the causal nature of the association between childhood physical abuse and incident metabolic syndrome during mid-life aging. Childhood abuse was measured after the time frame for measuring metabolic syndrome. However, the CTQ refers to experiences that occurred before the age 18. Scher and colleagues (2001) presented normative CTQ data in a community sample of middle-aged women, which were similar to the means in our sample for emotional abuse (M = 6.6, SD = 3.7 vs. M = 7.8, SD = 4.1), physical abuse (M = 6.4, SD = 2.9 vs. M = 6.6, SD = 2.8), and sexual abuse (M = 5.7, SD = 2.5 vs. M = 6.4, SD = 3.7). It is unclear whether prospective reports would have yielded more valid information, given the target age of abuse and the biases of under-reporting ongoing abuse. Another limitation pertains to the fact that women had to be pre- or peri-menopausal when entering the study and those who experienced the menopause prior to age 42 would not have been eligible. Thus, the current findings cannot be generalized to women experiencing menopause earlier in the lifespan. The measure used to assess childhood abuse has good reliability and validity, but retrospective recall of temporally distant and emotionally painful events have the potential for distortion, possibly because of repression, denial, or current mood at time of recall (Briere, 1992).
To our knowledge, this is the first study to suggest that childhood abuse is a risk factor for metabolic syndrome during mid-life aging, which is a period of increased cardiovascular risk for women. Physical abuse appears to be particularly detrimental in predicting adulthood metabolic syndrome. Previous studies suggest that childhood abuse is associated with cardiovascular disease; future studies should test whether metabolic syndrome accounts for this relationship.
Acknowledgements
The Study of Women’s Health Across the Nation (SWAN) has grant support from the National Institutes of Health (NIH), DHHS, through the National Institute on Aging (NIA), the National Institute of Nursing Research (NINR), and the NIH Office of Research on Women’s Health (ORWH) (Grants NR004061; AG012505, AG012535, AG012531, AG012539, AG012546, AG012553, AG012554, AG012495). Supplemental funding from the National Institute of Mental Health (MH59689) is also gratefully acknowledged. The content of this manuscript is solely the responsibility of the authors and does not necessarily represent the official views of the NIA, NINR, ORWH, or the NIH.
Footnotes
Participating institutions and principal staff were as follows. Clinical Center: University of Pittsburgh, Pittsburgh, PA - Karen Matthews, PI. NIH Program Office: National Institute on Aging, Bethesda, MD - Marcia Ory 1994 – 2001; Sherry Sherman 1994 – present; National Institute of Nursing Research, Bethesda, MD – Program Officers. Central Laboratory: University of Michigan, Ann Arbor - Daniel McConnell (Central Ligand Assay Satellite Services). Coordinating Center: New England Research Institutes, Watertown, MA - Sonja McKinlay, PI 1995 – 2001; University of Pittsburgh, Pittsburgh, PA – Kim Sutton-Tyrrell, PI 2001 – present. Steering Committee Chairs: Chris Gallagher and Susan Johnson. We thank the study staff and all the women who participated in SWAN.
Contributor Information
Aimee J. Midei, Department of Psychology, University of Pittsburgh
Karen A. Matthews, Department of Psychiatry, Psychology, and Epidemiology, University of Pittsburgh
Yue-Fang Chang, Department of Neurological Surgery, University of Pittsburgh
Joyce T. Bromberger, Department of Epidemiology and Psychiatry, University of Pittsburgh
References
- Ainsworth BE, Sternfeld B, Richardson MT, Jackson K. Evaluation of the Kaiser Physical Activity Survey in women. Medicine & Science in Sports & Exercise. 2000;32:1327–1338. doi: 10.1097/00005768-200007000-00022. [DOI] [PubMed] [Google Scholar]
- Allison PD. Survival analysis using SAS: A practical guide. Cary, NJ: SAS Institute Inc.; 2010. [Google Scholar]
- Baecke JA, Burema J, Frijters JE. A short questionnaire for the measurement of habitual physical activity in epidemiological studies. American Journal of Clinical Nutrition. 1982;36:936–942. doi: 10.1093/ajcn/36.5.936. [DOI] [PubMed] [Google Scholar]
- Bernstein DP, Fink L. Childhood Trauma Questionnaire. San Antonio, TX: Psychological Corp.; 1998. [Google Scholar]
- Bernstein DP, Fink L, Handelsman L, Foote J, Lovejoy M, Wenzel K, Ruggiero J. Initial reliability and validity of a new retrospective measure of child abuse and neglect. American Journal of Psychiatry. 1994;151:1132–1136. doi: 10.1176/ajp.151.8.1132. [DOI] [PubMed] [Google Scholar]
- Bernstein DP, Stein JA, Newcomb MD, Walker E, Pogge D, Ahluvalia T, Zule W. Development and validation of a brief screening version of the Childhood Trauma Questionnaire. Child Abuse & Neglect. 2003;27:169–190. doi: 10.1016/s0145-2134(02)00541-0. [DOI] [PubMed] [Google Scholar]
- Brewin CR, Andrews B, Valentine JD. Meta-analysis of risk factors for posttraumatic stress disorder in trauma-exposed adults. Journal of Consulting and Clinical Psychology. 2000;68:748–766. doi: 10.1037//0022-006x.68.5.748. [DOI] [PubMed] [Google Scholar]
- Briere J. Methodological issues in the study of sexual abuse effects. Journal of Consulting and Clinical Psychology. 1992;60:196–203. doi: 10.1037//0022-006x.60.2.196. [DOI] [PubMed] [Google Scholar]
- Cicchetti D, Rogosch FA. Diverse patterns of neuroendocrine activity in maltreated children. Development and Psychopathology. 2001;13:677–693. doi: 10.1017/s0954579401003145. [DOI] [PubMed] [Google Scholar]
- Danese A, Pariante CM, Caspi A, Taylor A, Poulton R. Childhood maltreatment predicts adult inflammation in a life-course study. PNAS. 2007;104:1319–1324. doi: 10.1073/pnas.0610362104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dong M, Anda RF, Felitti VJ, Dube SR, Williamson DF, Thompson TJ, Giles WH. The interrelatedness of multiple forms of childhood abuse, neglect, and household dysfunction. Child Abuse & Neglect. 2004;28:771–784. doi: 10.1016/j.chiabu.2004.01.008. [DOI] [PubMed] [Google Scholar]
- Dong M, Giles WH, Felitti VJ, Dube SR, Williams JE, Chapman DP, Anda RF. Insights into causal pathways for ischemic heart disease. Circulation. 2004;110:1761–1766. doi: 10.1161/01.CIR.0000143074.54995.7F. [DOI] [PubMed] [Google Scholar]
- Dube SR, Anda RF, Felitti VJ, Chapman DP, Williamson DF, Giles WH. Childhood abuse, household dysfunction, and the risk of attempted suicide throughout the life span: Findings from the Adverse Childhood Experiences Study. JAMA. 2001;286:3089–3096. doi: 10.1001/jama.286.24.3089. [DOI] [PubMed] [Google Scholar]
- Fechner-Bates S, Coyne JC, Schwenk TL. The relationship of self-reported distress to depressive disorders and other psychopathology. Journal of Consulting and Clinical Psychology. 1994;62:550–559. doi: 10.1037//0022-006x.62.3.550. [DOI] [PubMed] [Google Scholar]
- Felitti VJ, Anda RF, Nordenberg D, Williamson DF, Spitz AM, Edwards V, Marks JS. Relationship of childhood abuse and household dysfunction to many of the leading causes of death in adults. American Journal of Preventive Medicine. 1998;14:245–258. doi: 10.1016/s0749-3797(98)00017-8. [DOI] [PubMed] [Google Scholar]
- Finkelhor D, Ormrod RK, Turner HA. Re-victimization patterns in a national longitudinal sample of children and youth. Child Abuse & Neglect. 2007;31:479–502. doi: 10.1016/j.chiabu.2006.03.012. [DOI] [PubMed] [Google Scholar]
- Goldbacher EM, Matthews KA. Are psychological characteristics related to risk of the metabolic syndrome? A review of the literature. Annals of Behavioral Medicine. 2007;34:240–252. doi: 10.1007/BF02874549. [DOI] [PubMed] [Google Scholar]
- Greenfield EA, Marks NF. Violence from parents in childhood and obesity in adulthood: Using food in response to stress as a mediator of risk. [doi: DOI:10.1016/j.socscimed.2008.12.004] Soc Sci Med. 2009;68(5):791–798. doi: 10.1016/j.socscimed.2008.12.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gregoire F, Genart C, Hauser N, Remacle C. Glucocorticoids induce a drastic inhibition of proliferation and stimulate differentiation of adult rat fat cell precursors. Experimental Cell Research. 1991;196:270–278. doi: 10.1016/0014-4827(91)90261-r. [DOI] [PubMed] [Google Scholar]
- Grundy SM. The Metabolic Syndrome. In: Grundy SM, editor. Atlas of Atherosclerosis and Metabolic Syndrome. New York: Springer; 2011. pp. 1–26. [Google Scholar]
- Grundy SM, Cleeman JI, Daniels SR, Donato KA, Eckel RH, Franklin BA, Costa F. Diagnosis and Management of the Metabolic Syndrome: An American Heart Association/National Heart, Lung, and Blood Institute Scientific Statement: Executive Summary. Circulation. 2005;112:e285–e290. doi: 10.1161/CIRCULATIONAHA.105.169404. [DOI] [PubMed] [Google Scholar]
- Heim C, Newport DJ, Heit S, Graham YP, Wilcox M, Bonsall R, Nemeroff CB. Pituitary adrenal and autonomic responses to stress in women after sexual and physical abuse in childhood. Journal of the American Medical Association. 2000;284:592–597. doi: 10.1001/jama.284.5.592. [DOI] [PubMed] [Google Scholar]
- Lin J-W, Caffrey JL, Chang M-H, Lin Y-S. Sex, menopause, metabolic syndrome, and all-cause and cause-specific mortality--Cohort analysis from the Third National Health and Nutrition Examination Survey. Journal of Clinical Endocrinology and Metabolism. 2010;95:4258–4267. doi: 10.1210/jc.2010-0332. [DOI] [PubMed] [Google Scholar]
- Lorenzo C, Williams K, Hunt KJ, Haffner SM. The National Cholesterol Education Program–Adult Treatment Panel III, International Diabetes Federation, and World Health Organization Definitions of the metabolic syndrome as predictors of incident cardiovascular disease and diabetes. Diabetes Care. 2007;30:8–13. doi: 10.2337/dc06-1414. [DOI] [PubMed] [Google Scholar]
- Lundahl BW, Nimer J, Parsons B. Preventing child abuse: A meta-analysis of parent training programs. Res Social Work Prac. 2006;16:251–262. [Google Scholar]
- Matthews KAR, Katri, Gallo Linda, Kuller Lewis H. Association between socioeconomic status and metabolic syndrome in women: Testing the reserve capacity model. Health Psychology. 2008;27:576–583. doi: 10.1037/0278-6133.27.5.576. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Midei AJ, Matthews KA, Bromberger JT. Childhood abuse and neglect are associated with adiposity in mid-life women: A possible role for trait anger and reproductive hormones. Psychosomatic Medicine. 2010;72:215–223. doi: 10.1097/PSY.0b013e3181cb5c24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ninomiya JK, L'Italien G, Criqui MH, Whyte JL, Gamst A, Chen RS. Association of the metabolic syndrome with history of myocardial infarction and stroke in the Third National Health and Nutrition Examination Survey. Circulation. 2004;109:42–46. doi: 10.1161/01.CIR.0000108926.04022.0C. [DOI] [PubMed] [Google Scholar]
- Radloff LS. The CES-D Scale: A self-report depression scale for research in the general population. Applied Psychological Measurement. 1977;1:385–401. [Google Scholar]
- Rohde P, Ichikawa L, Simon GE, Ludman EJ, Linde JA, Jeffery RW, Operskalski BH. Associations of child sexual and physical abuse with obesity and depression in middle-aged women. Child Abuse Negl. 2008;32:878–887. doi: 10.1016/j.chiabu.2007.11.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scher CD, Stein MB, Asmundson GJG, McCreary DR, Forde DR. The Childhood Trauma Questionnaire in a community sample: psychometric properties and normative data. Journal of Traumatic Stress. 2001;14:843–857. doi: 10.1023/A:1013058625719. [DOI] [PubMed] [Google Scholar]
- Simpson TL, Miller WR. Concomitance between childhood sexual and physical abuse and substance use problems. A review. Clinical Psychology Reviews. 2002;22:27–77. doi: 10.1016/s0272-7358(00)00088-x. [DOI] [PubMed] [Google Scholar]
- Smith SCJ. Multiple risk factors for cardiovascular disease and diabetes mellitus. American Journal of Medicine. 2007;120:S3–S11. doi: 10.1016/j.amjmed.2007.01.002. [DOI] [PubMed] [Google Scholar]
- Spitzer RL, William JB, Gibbon M, First MB. The Structured Clinical Interview for DSM-III-R (SCID). I : History, rationale, and description. Archives of General Psychiatry. 1992;49:624–629. doi: 10.1001/archpsyc.1992.01820080032005. [DOI] [PubMed] [Google Scholar]
- Sternfeld B, Ainsworth BA, Quesenberry CPJ. Physical activity patterns in a diverse population of women. Preventative Medicine. 1999;28:313–323. doi: 10.1006/pmed.1998.0470. [DOI] [PubMed] [Google Scholar]
- Walker EA, Gelfan A, Katon WJ, Koss MP, Von Korff M, Bernstein D, Russo J. Adult health status of women with histories of childhood abuse and neglect. American Journal of Medicine. 1999;107:332–339. doi: 10.1016/s0002-9343(99)00235-1. [DOI] [PubMed] [Google Scholar]
- Wegman HL, Stetler C. A meta-analytic review of the effects of childhood abuse on medical outcomes in adulthood. Psychosomatic Medicine. 2009;71:805–812. doi: 10.1097/PSY.0b013e3181bb2b46. [DOI] [PubMed] [Google Scholar]
- Widom CS, Dumont KA, Czaja SJ. A prospective investigation of major depressive disorder and comorbidity in abused and neglected children grown up. Archives of General Psychiatry. 2007;64:49–56. doi: 10.1001/archpsyc.64.1.49. [DOI] [PubMed] [Google Scholar]