Highlight
► Choline-O-sulfate (COS), an osmolyte, inhibits amyloid formation of hIAPP. ► COS is the most effective inhibitor of amyloid formation among structural analogs. ► The sulfate group appears to be involved in the inhibition.
Abbreviations: COS, choline-O-sulfate; hIAPP, human islet amyloid polypeptide; NDSB, non-detergent sulfobetaine; HFIP, 1,1,1,3,3,3-hexafluoro-2-propanol; ThT, thioflavin T; CD, circular dichroism; TEM, transmission electron microscopy
Keywords: Osmolyte, Choline-O-sulfate, Aggregation inhibitor, Amyloid formation, Islet amyloid polypeptide
Abstract
Choline-O-sulfate (2-(trimethylammonio)ethyl sulfate, COS) is a naturally occurring osmolyte that is synthesized by plants, lichens, algae, fungi, and several bacterial species. We examined the inhibitory effects of COS on amyloid formation of the human islet amyloid polypeptide (hIAPP or amylin) using a thioflavin T (ThT) fluorescence assay, circular dichroism spectroscopy and transmission electron microscopy. The results showed that COS suppresses a conformational change of hIAPP from a random coil to a β-sheet structure, resulting in the inhibition of amyloid formation. Comparisons with various structural analogs including carnitine, acetylcholine and non-detergent sulfobetaines (NDSBs) using the ThT fluorescence assay showed that COS is the most effective inhibitor of hIAPP amyloid formation, suggesting that the sulfate group, which is unique to COS, significantly contributes to the inhibition.
1. Introduction
Osmolytes are small organic compounds that accumulate in cells in response to osmotic stress to prevent the misfolding/denaturation of proteins and ensure that they maintain their native structure [1]. Thus, osmolytes are often termed “chemical chaperones” or “small stress molecules”. Most of the organic osmolytes bear no net charge at physiological pH, and at high concentrations do not affect cytoplasmic functions such as protein catalysts and show no toxicity to the cellular environment.
These properties of osmolytes have enabled their application in biotechnology and medicine. Glycine betaine is a strong stabilizer of globular proteins against thermodenaturation or salt stress in vitro [2–4]. In contrast, destabilizing osmolytes such as arginine and lysine are commonly used to solubilize inclusion bodies and insoluble protein aggregates. Glycine betaine is often used as PCR enhancing agents that improve yields and the specificity of difficult targets (for example, GC-rich sequences) in PCR amplification reactions [5–8]. Osmolytes are capable of stabilizing and destabilizing proteins and DNA, depending on the osmolyte concentrations and/or solvent conditions (pH) [9,10]. In addition, several osmolytes, including trehalose [11], α-d-mannosylglycerate [12] and ectoine [13], are effective in preventing amyloid formation of Alzheimer’s Aβ peptides in vitro. Since these osmolytes are not toxic to the cellular environment, they represent potential inhibitors of neurodegenerative disorders [14].
This study focuses on the choline ester, choline-O-sulfate (2-(trimethylammonio)ethyl sulfate, COS), which is an osmolyte widely distributed in nature (Fig. 1). When compared with other osmolytes, the sulfate group of COS is unique and is the most characteristic structural and functional feature. COS is synthesized by a variety of plants, lichens, algae and fungi, and by several bacterial species [15–19]. The synthesis of COS is catalyzed by sulfur transferases that use 3′-phosphoadenosine-5′-phosphosulfate and choline as their substrates. COS also plays an important role in the microbial transformation of sulfur in the soil. Production of COS by the conjugation of sulfate with choline is proposed to serve in the detoxification of , whereas COS can also function as a source of choline and sulfur after hydrolysis by choline sulfatases [20]. The osmoprotective effects of COS are known for several plant, fungal and bacterial species, such as Halomonas elongata [21], Salmonella typhimurium [22], Limonium [17], Penicillium fellutanum [23], Escherichia coli and Bacillus subtilis [19]. However, the application of COS as an anti-aggregation reagent has not been reported.
Fig. 1.
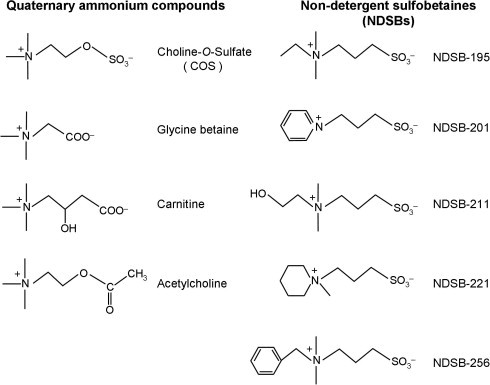
Chemical structure of COS and the structural analogs used in this study.
In this study, we examined the inhibitory effects of COS on amyloid formation of an amyloidogenic peptide associated with diseases using a thioflavin T (ThT) fluorescence assay, circular dichroism (CD) spectroscopy and transmission electron microscopy (TEM). The amyloid-forming peptide used was human islet amyloid polypeptide (hIAPP, also known as amylin), which is a 37-residue hormone peptide that is co-secreted with insulin from pancreatic β-cells [24,25]. In most type-2 diabetes patients, hIAPP is found in large deposits in the pancreas where it aggregates to form amyloid fibers. hIAPP aggregation is believed to be pathologically associated with β-cells toxicity in type-2 diabetes. In addition, to clarify which group of COS is functionally important in inhibiting amyloid formation, we used the ThT fluorescence assay to compare the activities of COS and various structural analogs, including glycine betaine, carnitine, acetylcholine and non-detergent sulfobetaines (NDSBs) (Fig. 1). Although NDSBs are not naturally synthesized, they are similar to COS in terms of quaternary ammonium compounds with a short hydrophobic group. NDSBs have often been used as agents to prevent protein aggregation and improve the yield of active proteins when added to buffers during in vitro protein renaturation [26–28]. A key difference between COS and NDSBs is that NDSBs have a sulfonate group, whereas COS has a sulfate group (Fig. 1). This is the first report describing the anti-aggregation properties of COS. The results show that COS is the most effective inhibitor of amyloid formation of hIAPP in vitro, with the sulfate group playing a key role in the inhibition.
2. Material and methods
2.1. Materials
Full-length hIAPP (37 residues) was purchased from the Peptide Institute, Inc. (Osaka, Japan). COS was obtained from SI Science Co., Ltd. (Tokyo, Japan). All NDSBs were purchased from Merck Japan Ltd. (Tokyo, Japan). 1,1,1,3,3,3-hexafluoro-2-propanol (HFIP) was purchased from Tokyo Chemical Industry Co., Ltd. (Tokyo, Japan). The other chemicals and reagents were purchased from Wako Pure Chemical Industries Ltd. (Osaka, Japan).
2.2. Preparation of peptide solutions
The preparation of synthetic hIAPP in a stable conformation was carried out according to Higham et al. [29]. hIAPP was dissolved in 100% HFIP to a concentration of 0.37 mg/ml and sonicated in a water bath for 2 min. The dissolved peptide was filtered (0.2 μm), aliquoted into microtubes, dried under vacuum and stored at −30 °C. Immediately prior to use, the HFIP-treated hIAPP was dissolved to a final concentration of 1 mM in 100% HFIP and diluted to a final concentration of 10 μM in 10 mM sodium acetate buffer (pH 6.4). Amyloid formation of hIAPP was followed by incubation at room temperature (25 °C).
2.3. The ThT fluorescence assay
The hIAPP sample solution was prepared as described above; the concentration was 10 μM hIAPP, 10 mM sodium acetate (pH 6.4) and 1% HFIP (v/v) in the absence of COS, or in the presence of COS or the structural analogs. For the assay, the ThT solution was added to the sample to a final concentration of 20 μM. ThT fluorescence changes were measured at room temperature using an FP-6500 spectrofluorometer (Jasco, Japan). The fluorescence intensity was monitored at an excitation wavelength of 450 nm and an emission wavelength of 482 nm with excitation and emission slit widths at 5 nm each. The curve fitting was according to a four-parameter sigmoidal curve using Sigma Plot v. 6.00 (SSPS Inc., USA) using the following equation:
| (1) |
where y is the fluorescence at time x, y0 is the initial fluorescence value, x0 is the time when the fluorescence reaches 50% of its maximum value, and a is the maximal fluorescence at the stationary phase [30].
2.4. Transmission electron microscope (TEM)
TEM was used to visualize the hIAPP aggregates. The concentration of the sample solution was the same as described in the ThT fluorescence assay. After a 24-hour incubation period with or without 100 mM COS at room temperature, 20 μl of the hIAPP sample was applied to the Carbon-coated Formvar 200 mesh copper grids. After 30 s, excess fluid was removed, and grids were negatively stained with 2% aqueous uranyl acetate (5 μl) for 10 s and dried overnight. Stained grids were viewed with a JEM-2010 TEM (JEOL, Japan).
2.5. Circular dichroism (CD) spectroscopy
CD spectra in the far-UV light range (190–260 nm) at each time point were measured at room temperature with a model J-720 spectropolarimeter (Jasco), using a cylindrical quartz cell with a 0.1 or 0.5 mm path length. The concentration of the sample solution was the same as described in the ThT fluorescence assay. For each sample, four consecutive spectra were acquired and baseline-subtracted. Results were expressed as mean residue ellipticity. The secondary structure content was estimated from each spectrum using the CDPro software package [31,32]. A reference data set, SDP42(#6), was used in the CDPro analysis.
3. Results and discussion
3.1. ThT fluorescence assay
hIAPP is among the most amyloidogenic peptides known, and has a strong in vitro tendency to aggregate into fibrils [33–35]. To establish identical starting conditions for the series of experiments, HFIP-treated hIAPP peptides were used. Under these conditions, the peptide is soluble and adopts primarily a random conformation that undergoes a slow transition to the amyloidogenic β-sheet structure [29].
ThT exhibits a characteristic fluorescence spectral change upon binding amyloid fibrils [36]. The effect of COS on amyloid formation of hIAPP was examined by monitoring changes in the ThT fluorescence of hIAPP (10 μM) in the presence of different concentrations of COS. When hIAPP was incubated alone, a time-dependent increase in ThT fluorescence was observed and followed a sigmoidal curve (Fig. 2A). Such an observation is typical of amyloid formation with an initial lag phase and subsequent phases of elongation and saturation [37,38]. Co-incubation of hIAPP with COS inhibited the amyloid formation of hIAPP in a concentration-dependent manner (Fig. 2A). Fig. 2B indicates that the IC50 value is approximately 70 mM. These results indicate that COS inhibits hIAPP aggregation.
Fig. 2.
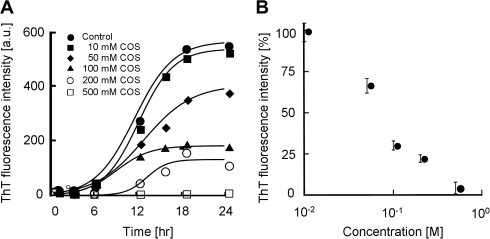
Effect of COS on hIAPP amyloid formation. (A) ThT fluorescence intensity of hIAPP (10 μM) at room temperature in the absence and presence of COS at concentrations from 10 to 500 mM. (B) Concentration-dependent inhibition by COS against hIAPP amyloid formation. Data are expressed as a ratio of the ThT fluorescence intensity of hIAPP incubated with COS at each concentration for 24 h to that of the control in the absence of COS. Values are the means ± standard deviations (n = 3). The IC50 values of COS were estimated to be approximately 70 mM.
3.2. TEM observations
TEM images showed that the control sample of the hIAPP aggregates in the absence of COS contained a high density of typical unbranched amyloid fibrils, which have been previously reported [35,39,40] (Fig. 3, left). In contrast, the samples in the presence of COS contained a much lower density of thin fibrils (Fig. 3, right). These results also demonstrated that COS prevents amyloid formation.
Fig. 3.
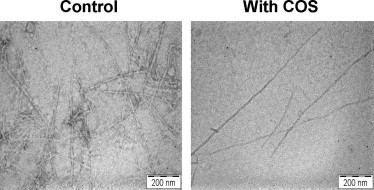
TEM images of hIAPP incubated without/with 100 mM COS for 24 h with 2% uranyl acetate. The COS concentrations approximately correspond to the IC50 values of COS.
3.3. CD spectral analysis
To examine how COS affects the secondary structure during hIAPP aggregation, we measured CD spectra at different time points in the presence or absence of COS. The hIAPP peptide in the absence of COS initially showed a CD spectrum with a minimum at 203 nm (Fig. 4A, left). Such a spectrum is characteristic of random coil conformations. CD spectra recorded at 30, 72 and 120 h demonstrated a time-dependent conversion from random coil to β-sheet conformation, as shown by a loss of the signal at 203 nm and the appearance of a minimum at 218 nm. According to the results of the CD deconvolution, the content of random coil conformation decreased from 64% to 34%, whereas the β-sheet content increased from 27% to 53% (Fig. 4B, left). The helical content did not significantly change. Similar results using CD analysis of the hIAPP peptide have been reported [35,39].
Fig. 4.
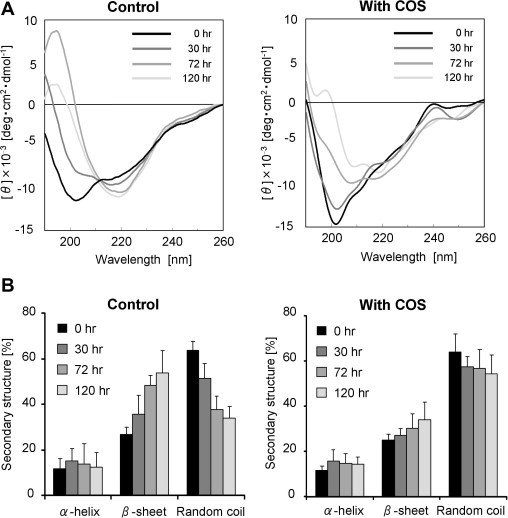
Effect of COS on the secondary structure of hIAPP during amyloid formation. (A) Far-UV CD spectroscopy of hIAPP recorded at room temperature at different time points in the absence (left) and presence (right) of 100 mM COS. Similar data were obtained in at least four replicate experiments. (B) Secondary structure prediction based on the CD spectra in the absence (left) and presence (right) of COS. Data are presented as mean ± standard deviation (n = 4).
In the presence of COS, however, the spectra remained primarily unchanged for the first 30 h, and therefore the peptide adopted a random coil conformation. At 72 h, the spectrum altered with minima at 207 and 219 nm (Fig. 4A, right). At 120 h, the amplitude of the spectrum had significantly decreased. This can be explained by the increase in insoluble amyloid fibrils of hIAPP as observed in the ThT fluorescence assay. The CD deconvolution indicated that the content of random coil decreased from 64% to 54%, and that of the β-sheet increased from 25% to 34% (Fig. 4B, right). Such an increase in β-sheet structure occurred more slowly when COS was present. With or without COS, the helical content remained at ∼15% for 120 h. Such virtually unchanged helical content over time is also observed in other similar in vitro experiments of hIAPP fibril formation [35,39]. These results showed that COS suppresses a conformational change of hIAPP from a random coil conformation to a β-sheet structure.
3.4. Comparison of the inhibitory effects with COS structural analogs
To examine which chemical group of COS functions more effectively in suppressing amyloid formation, we compared the inhibitory effects of COS with those of various structural analogs of COS using the ThT fluorescence assay. The analogues used included glycine betaine, carnitine, acetylcholine and five NDSBs (Fig. 1). The comparison showed that COS is the most effective inhibitor of hIAPP amyloid formation (Fig. 5). The inhibitory effects are in the order: COS > NDSB-195, -201, -211, -221 and -256 > acetylcholine, carnitine and glycine betaine. No significant difference among the NDSBs indicates that the distinct ‘side-chains’ grafted onto their quaternary amine are not directly involved in inhibiting hIAPP amyloid formation. Moreover, as COS was a more effective inhibitor than glycine betaine, acetylcholine or carnitine, all of which have the same quaternary amine group, this suggests that the sulfate group of COS is more important in suppressing amyloid formation than the other acid groups.
Fig. 5.
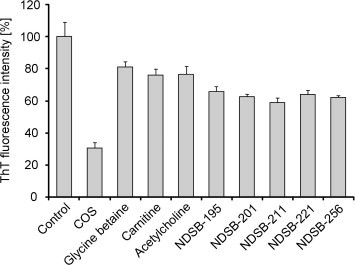
Inhibition of COS and the structural analogs on hIAPP amyloid formation. ThT fluorescence of hIAPP (10 μM) incubated with 100 mM COS or each structural analog was measured at room temperature after 24 h.
3.5. Concluding remarks
The results of this study showed that COS suppresses the structural conversion of hIAPP from a random coil conformation to a β-sheet structure, thereby inhibiting amyloid formation. Thus, the presence of COS appears to stabilize the random coil state of the peptide. Some osmolytes have both properties of stabilizing and destabilizing proteins, depending on the concentration and/or solvent conditions [41,42]. The stabilizing osmolytes are preferentially excluded from the immediate vicinity of the protein surface, and this exclusion suggests a solvophobic interaction between amino acids forming the protein surface and the protecting osmolytes. Conversely, certain solution conditions disfavor exclusion and this allows osmolytes to be preferentially bound to the protein, leading to the stabilization of the denatured protein state. Both functions are thought to involve the interaction of the osmolyte with the peptide bond [43–45]. Thus, presumably, COS preferentially interacts with the backbone of hIAPP and prevents interactions between the peptide backbone moieties that facilitate the formation of β-sheets and subsequent amyloid formation. The results in this study suggest that the sulfate group of COS efficiently interacts with the amide group of the peptide backbone. According to the TEM observations, the fibrils formed in the presence of COS, which were rarely observed, are thinner than the fibrils formed in the absence of COS. The putative interaction of COS with the peptides may have effects on the formation of the β-sheet structure and/or the fibrils.
The findings provide insights into the design of inhibitors against protein aggregation or amyloid formation; for example, a new NDSB containing a sulfate group. The application of COS or a new NDSB as an inhibitory agent against various kinds of aggregation states requires examination, and further studies will reveal the detailed molecular mechanism of the COS inhibition.
Acknowledgments
We thank Prof. Kenji Oosawa for providing access to the spectrofluorometer and Ms. Chiemi Ida for assistance with the operation of the TEM. We are grateful for the financial support from the JGC-S Scholarship Foundation (N.N.) and Gunma University Foundation for Science and Technology (N.N).
References
- 1.Kumar R. Role of naturally occurring osmolytes in protein folding and stability. Arch. Biochem. Biophys. 2009;491:1–6. doi: 10.1016/j.abb.2009.09.007. [DOI] [PubMed] [Google Scholar]
- 2.Caldas T., Demont-Caulet N., Ghazi A., Richarme G. Thermoprotection by glycine betaine and choline. Microbiology. 1999;145(Pt 9):2543–2548. doi: 10.1099/00221287-145-9-2543. [DOI] [PubMed] [Google Scholar]
- 3.Knapp S., Ladenstein R., Galinski E.A. Extrinsic protein stabilization by the naturally occurring osmolytes β-hydroxyectoine and betaine. Extremophiles. 1999;3:191–198. doi: 10.1007/s007920050116. [DOI] [PubMed] [Google Scholar]
- 4.Diamant S., Eliahu N., Rosenthal D., Goloubinoff P. Chemical chaperones regulate molecular chaperones in vitro and in cells under combined salt and heat stresses. J. Biol. Chem. 2001;276:39586–39591. doi: 10.1074/jbc.M103081200. [DOI] [PubMed] [Google Scholar]
- 5.Baskaran N., Kandpal R.P., Bhargava A.K., Glynn M.W., Bale A., Weissman S.M. Uniform amplification of a mixture of deoxyribonucleic acids with varying GC content. Genome Res. 1996;6:633–638. doi: 10.1101/gr.6.7.633. [DOI] [PubMed] [Google Scholar]
- 6.Hengen P.N. Optimizing multiplex and LA-PCR with betaine. Trends Biochem. Sci. 1997;22:225–226. doi: 10.1016/s0968-0004(97)01069-4. [DOI] [PubMed] [Google Scholar]
- 7.Henke W., Herdel K., Jung K., Schnorr D., Loening S.A. Betaine improves the PCR amplification of GC-rich DNA sequences. Nucleic Acids Res. 1997;25:3957–3958. doi: 10.1093/nar/25.19.3957. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Weissensteiner T., Lanchbury J.S. Strategy for controlling preferential amplification and avoiding false negatives in PCR typing. Biotechniques. 1996;21:1102–1108. doi: 10.2144/96216rr03. [DOI] [PubMed] [Google Scholar]
- 9.Natalello A., Liu J., Ami D., Doglia S.M., de Marco A. The osmolyte betaine promotes protein misfolding and disruption of protein aggregates. Proteins. 2009;75:509–517. doi: 10.1002/prot.22266. [DOI] [PubMed] [Google Scholar]
- 10.Singh L.R., Poddar N.K., Dar T.A., Kumar R., Ahmad F. Protein and DNA destabilization by osmolytes: the other side of the coin. Life Sci. 2011;88:117–125. doi: 10.1016/j.lfs.2010.10.020. [DOI] [PubMed] [Google Scholar]
- 11.Liu R., Barkhordarian H., Emadi S., Park C.B., Sierks M.R. Trehalose differentially inhibits aggregation and neurotoxicity of β-amyloid 40 and 42. Neurobiol. Dis. 2005;20:74–81. doi: 10.1016/j.nbd.2005.02.003. [DOI] [PubMed] [Google Scholar]
- 12.Ryu J., Kanapathipillai M., Lentzen G., Park C.B. Inhibition of β-amyloid peptide aggregation and neurotoxicity by α-d-mannosylglycerate, a natural extremolyte. Peptides. 2008;29:578–584. doi: 10.1016/j.peptides.2007.12.014. [DOI] [PubMed] [Google Scholar]
- 13.Kanapathipillai M., Lentzen G., Sierks M., Park C.B. Ectoine and hydroxyectoine inhibit aggregation and neurotoxicity of Alzheimer’s β-amyloid. FEBS Lett. 2005;579:4775–4780. doi: 10.1016/j.febslet.2005.07.057. [DOI] [PubMed] [Google Scholar]
- 14.Rajan R.S., Tsumoto K., Tokunaga M., Tokunaga H., Kita Y., Arakawa T. Chemical and pharmacological chaperones: application for recombinant protein production and protein folding diseases. Curr. Med. Chem. 2011;18:1–15. doi: 10.2174/092986711793979698. [DOI] [PubMed] [Google Scholar]
- 15.Catalfomo P., Block J.H., Constantine G.H., Kirk P.W. Choline sulfate (ester) in marine higher fungi. Mar. Chem. 1972;1:157–162. [Google Scholar]
- 16.Fitzgerald J.W., Luschinski P.C. Further studies on the formation of choline sulfate by bacteria. Can. J. Microbiol. 1977;23:483–490. doi: 10.1139/m77-072. [DOI] [PubMed] [Google Scholar]
- 17.Hanson A.D., Gage D.A. Identification and determination by fast atom bombardment mass spectrometry of the compatible solute choline-O-sulphate in Limonium species and other halophytes. Aust. J. Plant Physiol. 1991;18:317–327. [Google Scholar]
- 18.Hanson A.D., Rathinasabapathi B., Chamberlin B., Gage D.A. Comparative physiological evidence that β-alanine betaine and choline-O-sulfate act as compatible osmolytes in halophytic Limonium species. Plant Physiol. 1991;97:1199–1205. doi: 10.1104/pp.97.3.1199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Hanson A.D., Rathinasabapathi B., Rivoal J., Burnet M., Dillon M.O., Gage D.A. Osmoprotective compounds in the Plumbaginaceae: a natural experiment in metabolic engineering of stress tolerance. Proc. Natl. Acad. Sci. USA. 1994;91:306–310. doi: 10.1073/pnas.91.1.306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Markham P., Robson G.D., Bainbridge B.W., Trinci A.P. Choline: its role in the growth of filamentous fungi and the regulation of mycelial morphology. FEMS Microbiol. Rev. 1993;10:287–300. doi: 10.1111/j.1574-6968.1993.tb05872.x. [DOI] [PubMed] [Google Scholar]
- 21.Cánovas D., Vargas C., Csonka L.N., Ventosa A., Nieto J.J. Osmoprotectants in Halomonas elongata: high-affinity betaine transport system and choline-betaine pathway. J. Bacteriol. 1996;178:7221–7226. doi: 10.1128/jb.178.24.7221-7226.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Gutierrez J.A., Csonka L.N. Isolation and characterization of adenylate kinase (adk) mutations in Salmonella typhimurium which block the ability of glycine betaine to function as an osmoprotectant. J. Bacteriol. 1995;177:390–400. doi: 10.1128/jb.177.2.390-400.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Park Y.I., Gander J.E. Choline derivatives involved in osmotolerance of Penicillium fellutanum. Appl. Environ. Microbiol. 1998;64:273–278. doi: 10.1128/aem.64.1.273-278.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Khemtemourian L., Killian J.A., Hoppener J.W., Engel M.F. Recent insights in islet amyloid polypeptide-induced membrane disruption and its role in β-cell death in type 2 diabetes mellitus. Exp. Diabetes Res. 2008;2008:421287. doi: 10.1155/2008/421287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Westermark P., Andersson A., Westermark G.T. Islet amyloid polypeptide, islet amyloid, and diabetes mellitus. Physiol. Rev. 2011;91:795–826. doi: 10.1152/physrev.00042.2009. [DOI] [PubMed] [Google Scholar]
- 26.Vuillard L., Braun-Breton C., Rabilloud T. Non-detergent sulphobetaines: a new class of mild solubilization agents for protein purification. Biochem. J. 1995;305(Pt 1):337–343. doi: 10.1042/bj3050337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Expert-Bezançon N., Rabilloud T., Vuillard L., Goldberg M.E. Physical-chemical features of non-detergent sulfobetaines active as protein-folding helpers. Biophys. Chem. 2003;100:469–479. doi: 10.1016/s0301-4622(02)00299-5. [DOI] [PubMed] [Google Scholar]
- 28.Xiang L., Ishii T., Hosoda K., Kamiya A., Enomoto M., Nameki N., Inoue Y., Kubota K., Kohno T., Wakamatsu K. Interaction of anti-aggregation agent dimethylethylammonium propane sulfonate with acidic fibroblast growth factor. J. Magn. Reson. 2008;194:147–151. doi: 10.1016/j.jmr.2008.06.006. [DOI] [PubMed] [Google Scholar]
- 29.Higham C.E., Jaikaran E.T., Fraser P.E., Gross M., Clark A. Preparation of synthetic human islet amyloid polypeptide (IAPP) in a stable conformation to enable study of conversion to amyloid-like fibrils. FEBS Lett. 2000;470:55–60. doi: 10.1016/s0014-5793(00)01287-4. [DOI] [PubMed] [Google Scholar]
- 30.Arora A., Ha C., Park C.B. Inhibition of insulin amyloid formation by small stress molecules. FEBS Lett. 2004;564:121–125. doi: 10.1016/S0014-5793(04)00326-6. [DOI] [PubMed] [Google Scholar]
- 31.Sreerama N., Venyaminov S.Y., Woody R.W. Estimation of protein secondary structure from circular dichroism spectra: inclusion of denatured proteins with native proteins in the analysis. Anal. Biochem. 2000;287:243–251. doi: 10.1006/abio.2000.4879. [DOI] [PubMed] [Google Scholar]
- 32.Sreerama N., Woody R.W. Estimation of protein secondary structure from circular dichroism spectra: comparison of CONTIN, SELCON, and CDSSTR methods with an expanded reference set. Anal. Biochem. 2000;287:252–260. doi: 10.1006/abio.2000.4880. [DOI] [PubMed] [Google Scholar]
- 33.Goldsbury C., Kistler J., Aebi U., Arvinte T., Cooper G.J. Watching amyloid fibrils grow by time-lapse atomic force microscopy. J. Mol. Biol. 1999;285:33–39. doi: 10.1006/jmbi.1998.2299. [DOI] [PubMed] [Google Scholar]
- 34.Goldsbury C.S., Cooper G.J., Goldie K.N., Muller S.A., Saafi E.L., Gruijters W.T., Misur M.P., Engel A., Aebi U., Kistler J. Polymorphic fibrillar assembly of human amylin. J. Struct. Biol. 1997;119:17–27. doi: 10.1006/jsbi.1997.3858. [DOI] [PubMed] [Google Scholar]
- 35.Kayed R., Bernhagen J., Greenfield N., Sweimeh K., Brunner H., Voelter W., Kapurniotu A. Conformational transitions of islet amyloid polypeptide (IAPP) in amyloid formation in vitro. J. Mol. Biol. 1999;287:781–796. doi: 10.1006/jmbi.1999.2646. [DOI] [PubMed] [Google Scholar]
- 36.Biancalana M., Koide S. Molecular mechanism of Thioflavin-T binding to amyloid fibrils. Biochim. Biophys. Acta. 2010;1804:1405–1412. doi: 10.1016/j.bbapap.2010.04.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Gosal W.S., Morten I.J., Hewitt E.W., Smith D.A., Thomson N.H., Radford S.E. Competing pathways determine fibril morphology in the self-assembly of β2-microglobulin into amyloid. J. Mol. Biol. 2005;351:850–864. doi: 10.1016/j.jmb.2005.06.040. [DOI] [PubMed] [Google Scholar]
- 38.Nielsen L., Khurana R., Coats A., Frokjaer S., Brange J., Vyas S., Uversky V.N., Fink A.L. Effect of environmental factors on the kinetics of insulin fibril formation: elucidation of the molecular mechanism. Biochemistry. 2001;40:6036–6046. doi: 10.1021/bi002555c. [DOI] [PubMed] [Google Scholar]
- 39.Goldsbury C., Goldie K., Pellaud J., Seelig J., Frey P., Muller S.A., Kistler J., Cooper G.J., Aebi U. Amyloid fibril formation from full-length and fragments of amylin. J. Struct. Biol. 2000;130:352–362. doi: 10.1006/jsbi.2000.4268. [DOI] [PubMed] [Google Scholar]
- 40.Porat Y., Mazor Y., Efrat S., Gazit E. Inhibition of islet amyloid polypeptide fibril formation: a potential role for heteroaromatic interactions. Biochemistry. 2004;43:14454–14462. doi: 10.1021/bi048582a. [DOI] [PubMed] [Google Scholar]
- 41.Arakawa T., Timasheff S.N. The stabilization of proteins by osmolytes. Biophys. J. 1985;47:411–414. doi: 10.1016/S0006-3495(85)83932-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Timasheff S.N. Protein-solvent preferential interactions, protein hydration, and the modulation of biochemical reactions by solvent components. Proc. Natl. Acad. Sci. USA. 2002;99:9721–9726. doi: 10.1073/pnas.122225399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Auton M., Bolen D.W. Additive transfer free energies of the peptide backbone unit that are independent of the model compound and the choice of concentration scale. Biochemistry. 2004;43:1329–1342. doi: 10.1021/bi035908r. [DOI] [PubMed] [Google Scholar]
- 44.Liu Y., Bolen D.W. The peptide backbone plays a dominant role in protein stabilization by naturally occurring osmolytes. Biochemistry. 1995;34:12884–12891. doi: 10.1021/bi00039a051. [DOI] [PubMed] [Google Scholar]
- 45.Bolen D.W., Baskakov I.V. The osmophobic effect: natural selection of a thermodynamic force in protein folding. J. Mol. Biol. 2001;310:955–963. doi: 10.1006/jmbi.2001.4819. [DOI] [PubMed] [Google Scholar]


