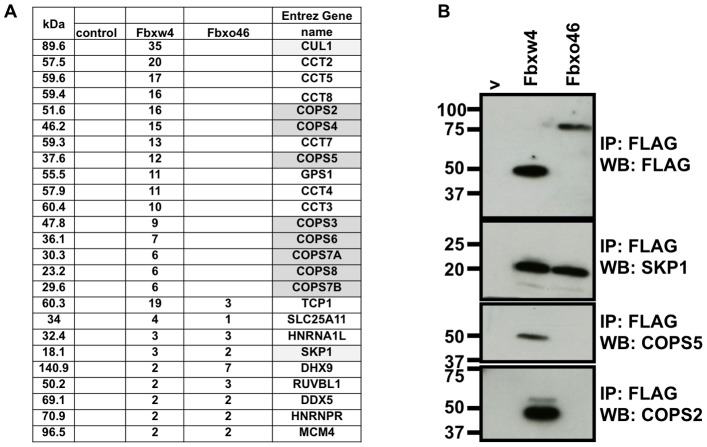
Figure 2. Fbxw4 interacts with components of an E3 ubiquitin ligase complex and the COP9 signalosome.
A. Table representing the number of unique peptides identified from one representative mass spectrometry experiment following FLAG immunoprecipitation from lysates of control cells expressing FLAG only “contr.”, or cells expressing FLAG-Fbxw4 or FLAG-Fbxo46. Column on left indicates the size of the interacting protein, in kilo-daltons (kDa). Gene names of proteins that contain the identified peptides are shown in the right column. Components of an E3 ubiquitin ligase complex are shaded in light gray; components of the COP9 signalosome are shaded dark gray. B. Validation of data mass spectrometry data by immunoprecipitation followed by western blot. 293 T cells were transfected with plasmids containing FLAG- Fbxw4, FLAG-Fbxo46 or an empty vector (v). 48 hours post-transfection cell lysates were prepared and immunoprecipitations were performed with mono-clonal anti-FLAG antibodies (M2) (to immunoprecipitate Fbxw4- or Fbxo46-interacting complexes). Western blots were performed to detect Fbxw4 or Fbxo46 (FLAG rb; polyclonal FLAG antibody; top panel), SKP1, COPS5, or COPS2. C. Expression of Fbxw4 alters the migration of endogenous SKP1 by gel filtration chromatography. 293 T cells were transfected with an empty vector (left panels) or a cplasmid containing FLAG-Fbxw4 (right panels). 48 hours post-transfection cell lysates were prepared and separated on a superpose6 gel filtration column. Western blots were performed on every other fraction to detect Fbxw4 (top panels) or SKP1 (bottom panels). In the absence of Fbxw4 SKP1 elutes with a peak at fraction 23, whereas when Fbxw4 is expressed there is co-elution of Fbxw4 with peaks at fraction 15 and in the void volume. Size standards that elute from given fractions are shown.